Method for preparing rapid detection test paper of novel coronavirus IgA antibody
A coronavirus and test strip technology, applied in the field of recombinant protein detection of novel coronavirus 2019-nCoV IgA antibodies, can solve the problems of lack of non-invasive, rapid and accurate screening of 2019 novel coronavirus infection, patient discomfort, cross-infection, etc. Achieve the effect of high-throughput detection, low detection environment requirements, and high fault tolerance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The invention provides the preparation method of described test strip, comprises the following steps:
[0038] 1) After the fluorescent microspheres are activated, they are mixed and coupled with the recombinant protein to obtain the 2019 novel coronavirus-specific antigen labeled with fluorescent microspheres;
[0039] 2) spraying the 2019 novel coronavirus-specific antigen labeled with fluorescent microspheres onto the glass fiber membrane to obtain a fluorescent microsphere pad;
[0040] 3) Anti-human IgA antibody and goat anti-chicken IgY antibody are sprayed respectively on the detection zone and the quality control zone of the nitrocellulose membrane to obtain the nitrocellulose membrane fixed with the detection zone and the quality control zone;
[0041] (4) Paste sample absorbent pads, fluorescent microsphere pads, nitrocellulose membranes fixed with detection areas and quality control areas, and water absorbent pads on the base plate in order to obtain test str...
Embodiment 1
[0052] Preparation of the novel coronavirus 2019-nCoV IgA antibody detection reagent strip.
[0053] 1. Preparation of novel coronavirus 2019-nCoV recombinant NP protein:
[0054] The new coronavirus 2019-nCoV recombinant NP protein has a cysteine residue added to the C-terminus, and the amino acid sequence is shown in the sequence Seq ID No.1. Entrusted Beijing Deoping Biotechnology Co., Ltd. to produce and prepare, the purity is greater than 95%.
[0055]MSDNGPQNQRNAPRITFGGPSDSTGSNQNGERSGARSKQRRPQGLP NNTASWFTALTQHGKEDLKFPRGQGVPINTNSSPDDQIGYYRRATRRIRG GDGKMKDLSPRWYFYYLGTGPEAGLPYGANKDGIIWVATEGALNTPKD HIGTRNPANNAAIVLQLPQGTTLPKGFYAEGSRGGSQASSRSSSRSRNSSR NSTPGSSRGTSPARMAGNGGDAALALLLLDRLNQLESKMSGKGQQQQG QTVTKKSAAEASKKPRQKRTATKAYNVTQAFGRRGPEQTQGNFGDQELIRQGTDYKHWPQIAQFAPSASAFFGMSRIGMEVTPSGTWLTYTGAIKLDD KDPNFKDQVILLNKHIDAYKTFPPTEPKKDKKKKADETQALPQRQKKQQ TVTLLPAADLDDFSKQLQQSMSSADSTQAGSSC
[0056] 2. Preparation of 2019-nCoV recombinant NP antigen labeled with fluorescent microsphere...
Embodiment 2
[0068] Application of time-resolved fluorescent immunochromatographic test strips for detection of novel coronavirus 2019-nCoV IgA antibody
[0069] 1. Sample pretreatment
[0070] Aspirate 20 μL saliva sample and add to 200 μL sample diluent, mix well.
[0071] 2. Test with test strips
[0072] Use a micropipette to accurately draw 80 μL of the sample solution to be tested into the sample hole of the test strip, and act for 15 minutes at room temperature (20-25°C); insert the test paper card into the carrier of the fluorescence detector, and select the sample to be tested through the touch screen. item, press the "Start Detection" button, the fluorescence detector will automatically scan the test paper card; read or print the test results on the display screen of the instrument.
[0073] 3. Analysis of test results
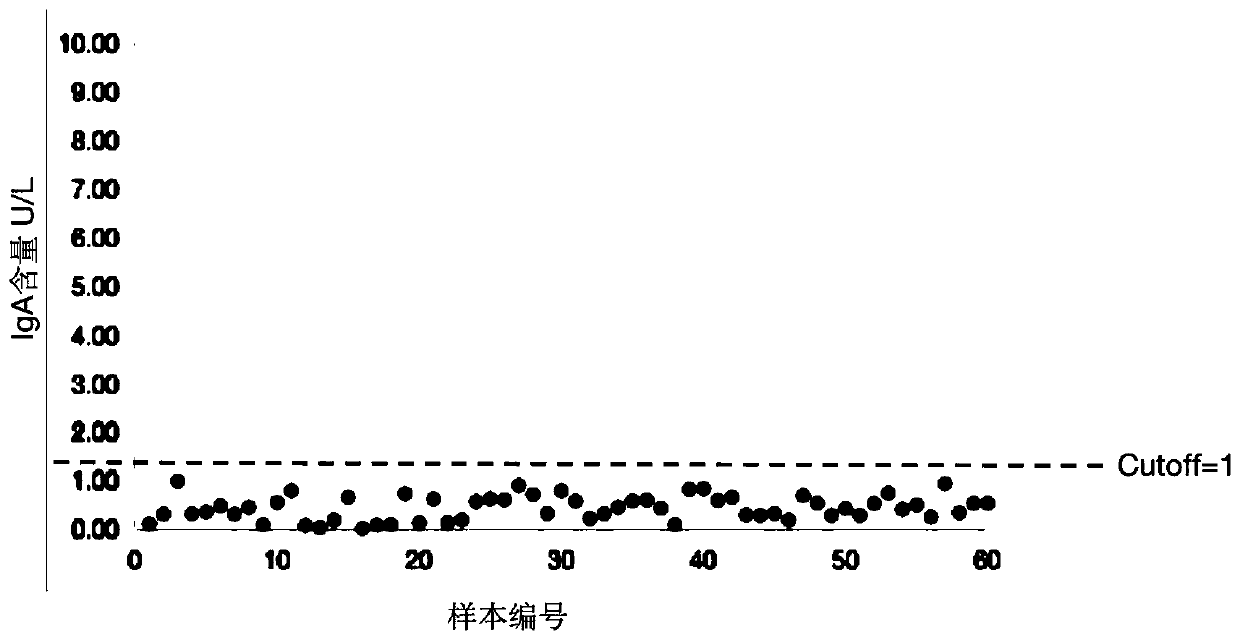
[0074] Quantitative detection
[0075] After the test is completed, the instrument obtains the ratio of the time-resolved fluorescence intensity of the detec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com