Drug combination composition for treating tumor diseases, and application thereof
A combined drug and composition technology, applied in the field of biomedicine, can solve the problem that there is no combination of anti-PD-1 monoclonal antibody and anti-VEGFR-2 antibody approved for marketing, so as to prolong the progression-free survival and overall survival , Improve the duration of therapeutic response, and the effect of high drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
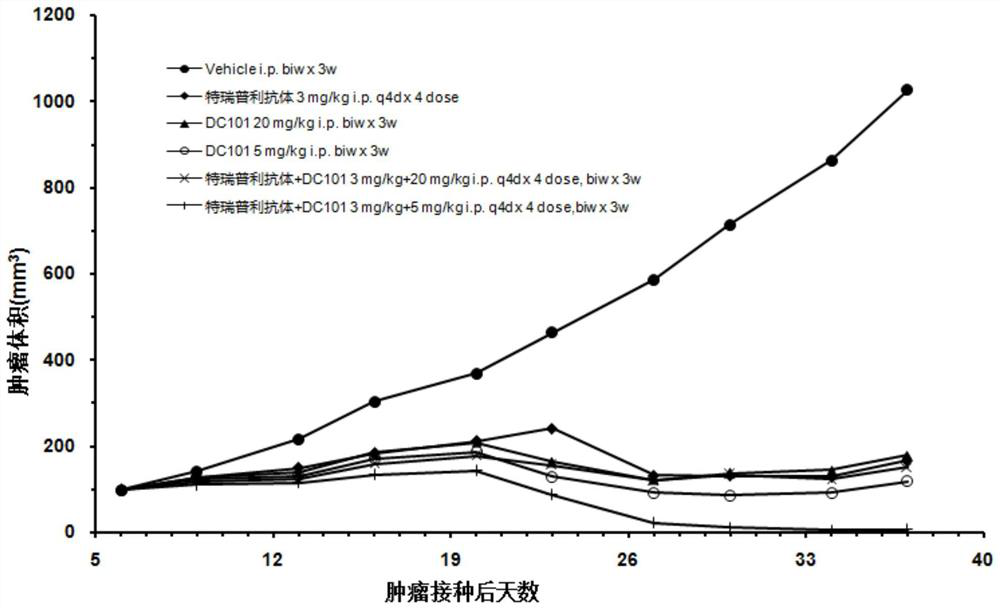
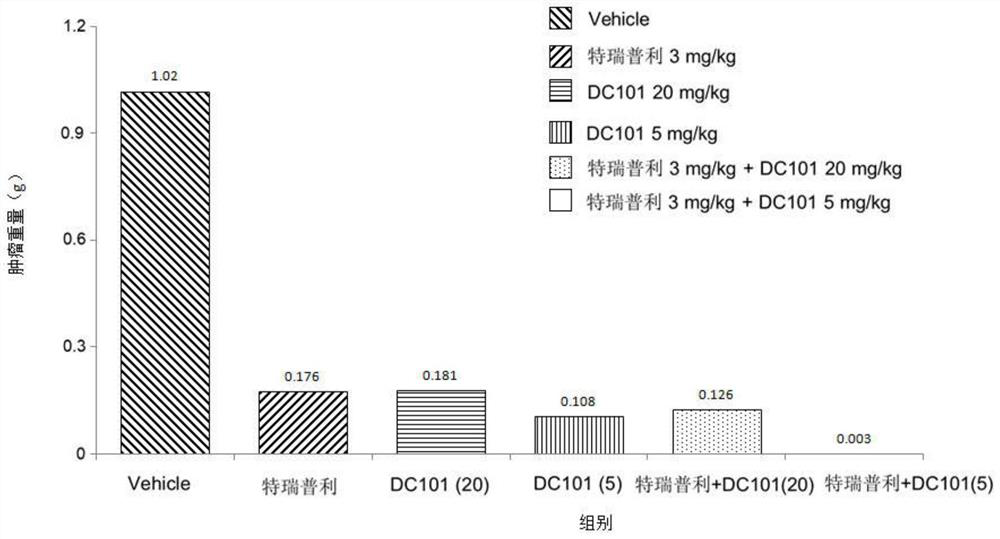
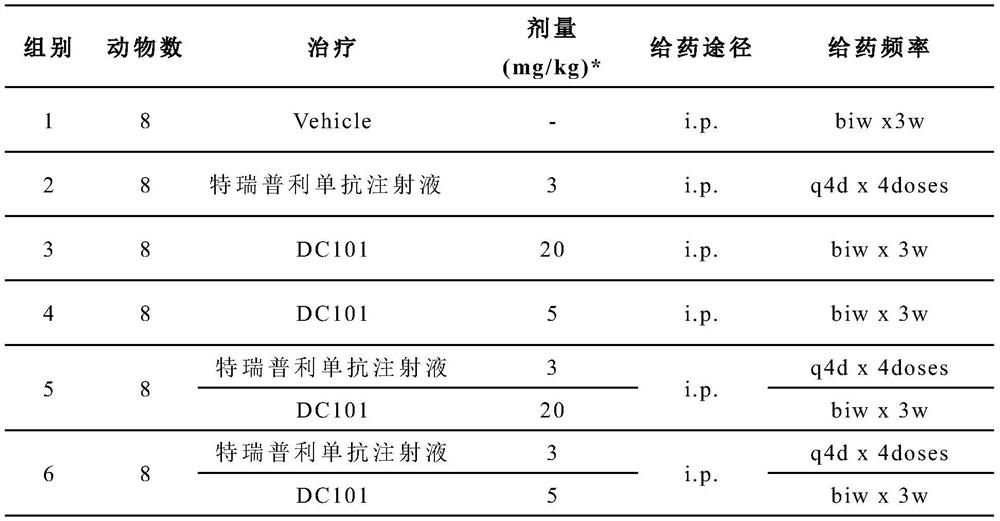
[0030] Example 1 of the present invention provides a combined drug composition for the treatment of tumor diseases, including an anti-PD-1 monoclonal antibody targeting PD-1 and an anti-VEGFR-2 monoclonal antibody targeting VEGFR-2 .
[0031] Further, anti-PD-1 monoclonal antibodies include DFPD1-9, DFPD1-10, DFPD1-11, DFPD1-12, DFPD1-13, Nivolumab, Pembrolizumab, toripalimab, sintilimab, tiram Rizumab, camrelizumab, pembrolizumab, or cepalimumab.
[0032] Among them, Nivolumab, Pembrolizumab, Toripalimab, Sintilimab, Tislelizumab, Camrelizumab, Pembrolizumab or Sepalimumab are all listed products .
[0033] Among them, DFPD1-9, DFPD1-10, DFPD1-11, DFPD1-12, and DFPD1-13 are the anti-PD-1 monoclonal antibodies provided by the application number CN201510312910.8, and DFPD1-9 includes as shown in SEQ ID No: 2 The light chain variable region shown and the heavy chain variable region shown in SEQ ID No: 1; DFPD1-10 includes the light chain variable region shown in SEQ ID No: 3 ...
Embodiment 2
[0061] On the basis of Example 1, Example 2 of the present invention further provides a combination drug composition for treating tumor diseases, including an anti-PD-1 monoclonal antibody targeting PD-1 and an anti-PD-1 monoclonal antibody targeting VEGFR-2 anti-VEGFR-2 monoclonal antibody.
[0062] Preferably, the anti-PD-1 monoclonal antibody is toripalimab.
[0063] Preferably, the anti-VEGFR-2 monoclonal antibody is antibody N-3, and antibody N-3 includes a light chain variable region as shown in SEQ ID No:8 and a heavy chain variable region as shown in SEQ ID No:9 .
[0064] Preferably, the administration of toripalimab and antibody N-3 is sequential.
[0065] The dosage of toripalimab is 100-400 mg, given once every 3 weeks.
[0066] The dosage of antibody N-3 was 4-20 mg / kg, given once every 3 weeks.
[0067] Both toripalimab and antibody N-3 were administered intravenously.
Embodiment 3
[0069] Example 3 of the present invention further provides a combination drug composition for treating tumor diseases on the basis of Example 2. The difference from Example 2 is that the dosage of toripalimab is 240 mg, given once every 3 weeks ; The dosage of antibody N-3 was 8 mg / kg, and other schemes were the same as in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com