Drug combination composition for treating tumor diseases and application
A combined drug and composition technology, applied in the field of biomedicine, can solve the problem of the lack of approval and marketing of the combined drug of anti-PD-1 monoclonal antibody and anti-EGFR monoclonal antibody, so as to prolong progression-free survival and overall survival, and improve treatment Effects on duration of response, improved health status, and quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
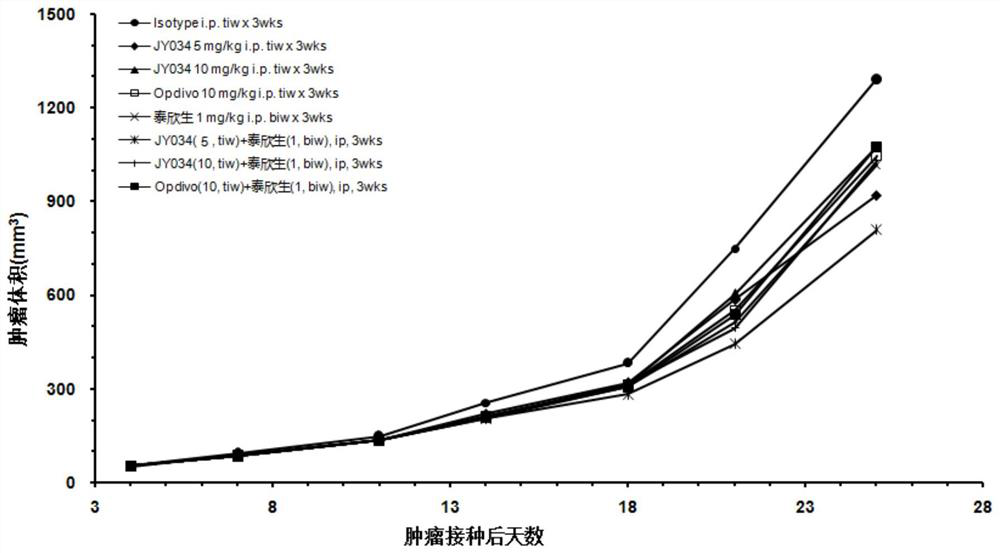
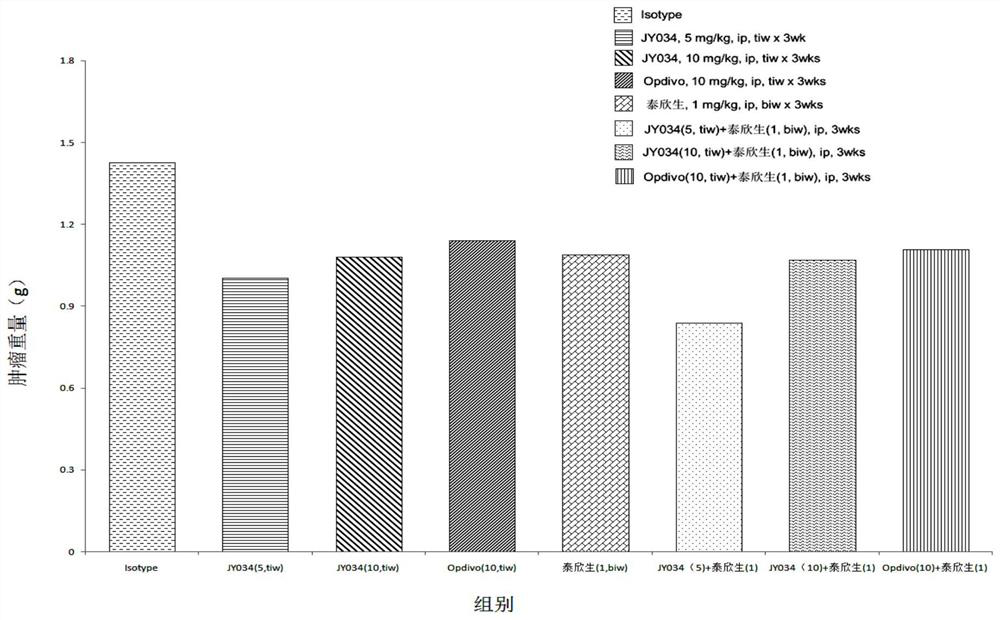
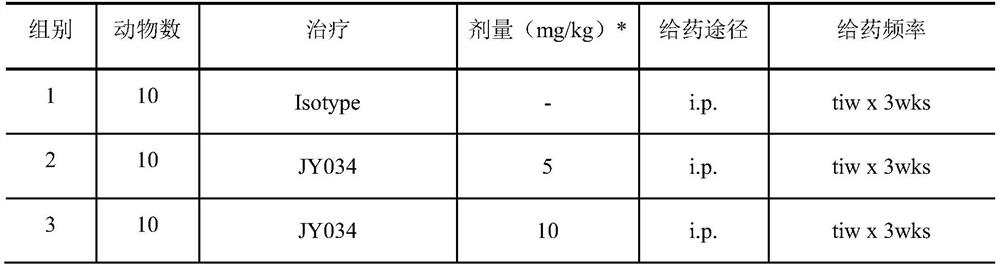
Image
Examples
Embodiment 1
[0028] Example 1 of the present invention provides a combination drug composition for treating tumor diseases, including an anti-PD-1 monoclonal antibody targeting PD-1 and an anti-EGFR monoclonal antibody targeting EGFR.
[0029] Anti-PD-1 monoclonal antibodies include DFPD1-9, DFPD1-10, DFPD1-11, DFPD1-12, DFPD1-13, Nivolumab, Pembrolizumab, toripalimab, sintilimab, tislelizumab Antibodies, camrelizumab, pembrolizumab, or cepalimumab.
[0030] Among them, Nivolumab, Pembrolizumab, Toripalimab, Sintilimab, Tislelizumab, Camrelizumab, Pembrolizumab or Sepalimumab are all listed products .
[0031] Among them, DFPD1-9, DFPD1-10, DFPD1-11, DFPD1-12, and DFPD1-13 are the anti-PD-1 monoclonal antibodies provided by the application number CN201510312910.8, and DFPD1-9 includes as shown in SEQ ID No: 2 The light chain variable region shown and the heavy chain variable region shown in SEQ ID No: 1; DFPD1-10 includes the light chain variable region shown in SEQ ID No: 3 and the heav...
Embodiment 2
[0047] On the basis of Example 1, Example 2 of the present invention provides a combination drug composition for treating tumor diseases, including an anti-PD-1 monoclonal antibody targeting PD-1 and an anti-EGFR targeting EGFR Monoclonal antibodies.
[0048] The anti-PD-1 monoclonal antibody is DFPD1-10, and DFPD1-10 includes a light chain variable region as shown in SEQ ID No:3 and a heavy chain variable region as shown in SEQ ID No:1.
[0049] The anti-EGFR monoclonal antibody is Nimotuzumab.
[0050] The administration of DFPD1-10 and Nimotuzumab was sequential.
[0051] Both DFPD1-10 and nimotuzumab were administered intravenously.
[0052] The dosage of DFPD1-10 is 100mg;
[0053] The dosage of Nimotuzumab is 100mg;
[0054] The dosing cycle of DFPD1-10 was every two weeks, and the dosing cycle of nimotuzumab was once a week.
Embodiment 3
[0056] Example 3 of the present invention further limits the dosage of DFPD1-10 to 200mg on the basis of Example 2;
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com