Genetically engineered oncolytic vaccinia viruses and methods of use thereof
A vaccinia virus and oncolytic technology, applied in genetic engineering, plant genetic improvement, virus, etc., can solve problems such as inability to exert therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
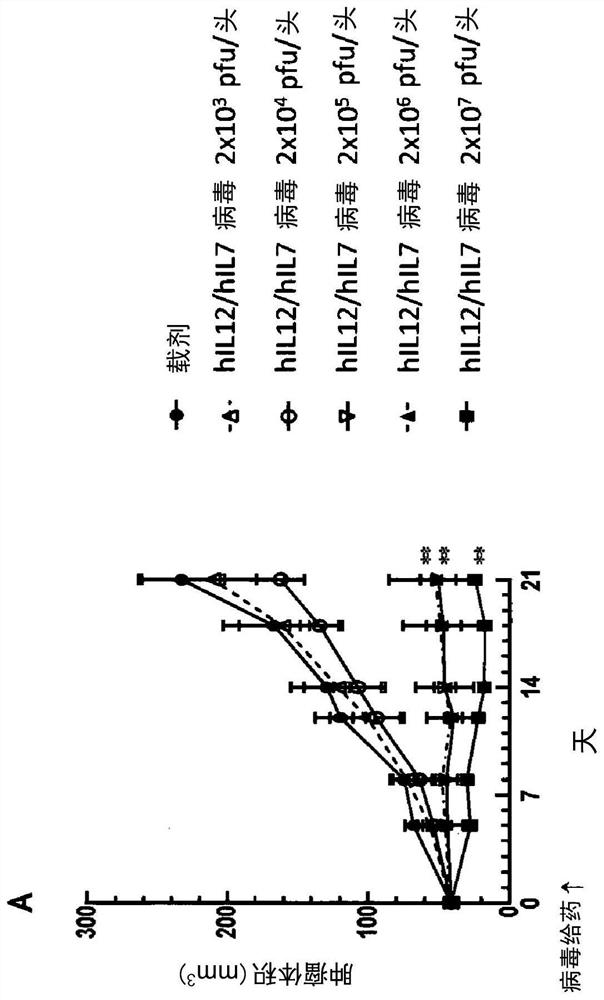
Examples
Embodiment 1
[0339] Example 1. Cytotoxicity of vaccinia virus carrying hIL12 and hIL7 on human tumor cells
[0340] This study was conducted to determine whether vaccinia virus carrying hIL12 and hIL7 showed cytotoxic effects in the following human cancer cell lines: human colorectal cancer (COLO 741) cells, human glioblastoma (U-87MG) cells and human cholangiocarcinoma (HuCCT1) cells.
[0341] All cells were infected with vaccinia virus carrying hIL12 and hIL7 at different multiplicities of infection (MOI) (0, 0.1, 1, 10 and 100). 45 days after infection, with CellTiter- The 2.0 assay measures cell viability. Cell viability was calculated by setting uninfected cells (MOI 0) and medium control wells without cells to 100% and 0% viability, respectively. One experiment was performed and data are presented as the mean of three replicate measurements.
[0342] At 4 days post-infection, cell viability dropped to <10% (Table 1). These results indicate that vaccinia viruses carrying hIL12 a...
Embodiment 2
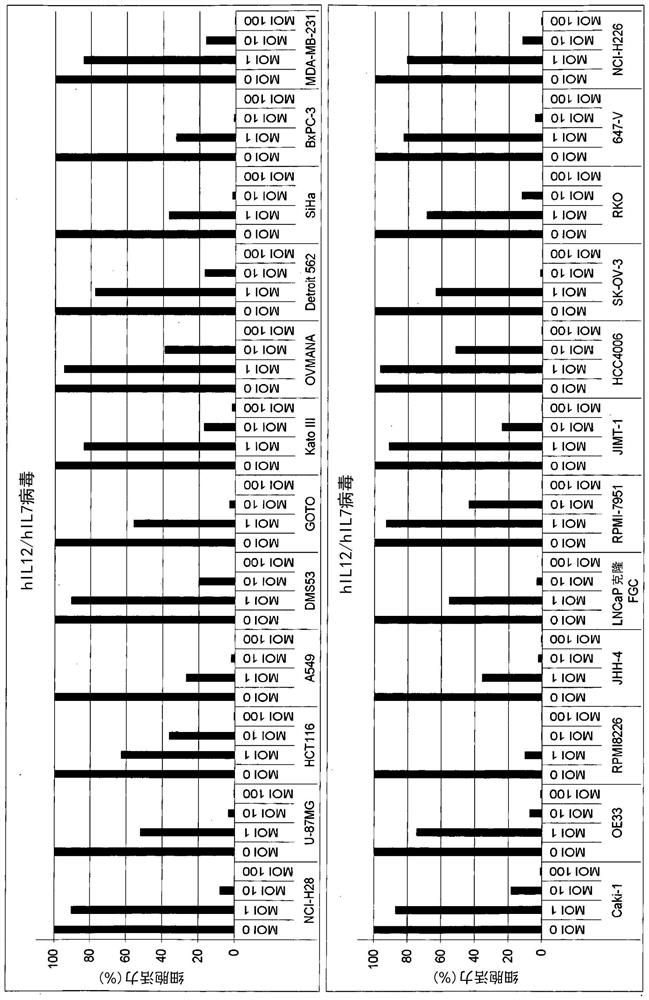
[0346] Example 2. Cytotoxic activity of vaccinia virus carrying hIL12 and hIL7 against various human cancer cell lines
[0347] This study was conducted to further examine whether hIL12 and hIL7-carrying vaccinia viruses exhibited cytotoxic effects on 24 human cancer cell lines.
[0348] Cells were infected with vaccinia virus carrying hIL12 and hIL7 at different multiplicities of infection (MOI). 5 days after infection, with CellTiter- The luminescent cell viability assay measures cell viability. Cell viability was calculated by setting uninfected cells (MOI 0) and medium control wells without cells to 100% and 0% viability, respectively. One experiment was performed and data are presented as the mean of three replicate measurements.
[0349] like figure 1 As shown, vaccinia viruses carrying hIL12 and hIL7 were cytotoxic to all human cancer cells examined 5 days after infection at MOIs of 1.0, 10 or 100.
Embodiment 3
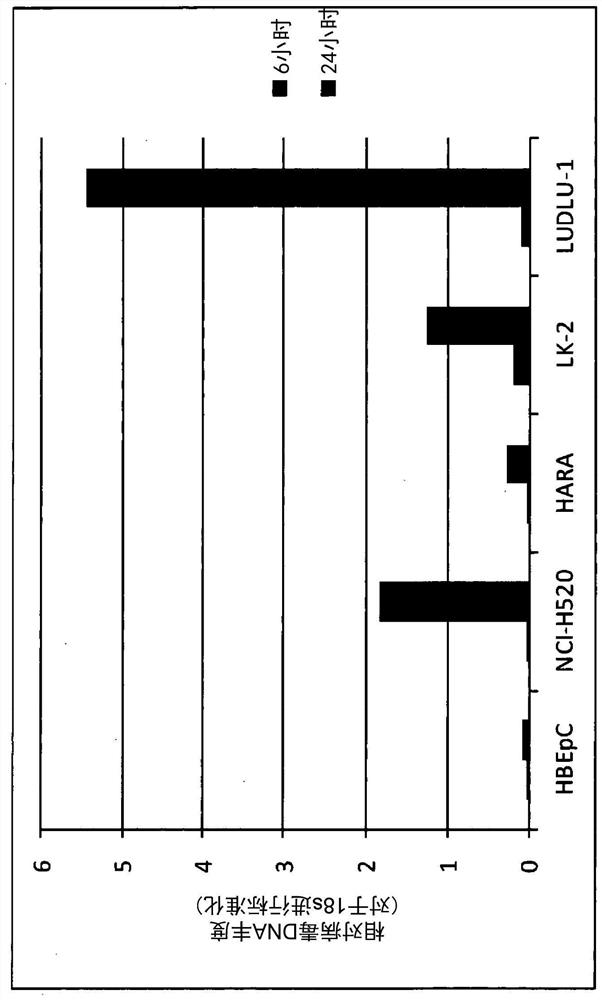
[0350] Example 3. Replication of vaccinia virus carrying hIL12 and hIL7 in human cancer cells or normal cells
[0351] This study was conducted to examine whether hIL12 and hIL7-carrying vaccinia viruses replicate selectively in human cancer cells relative to normal cells.
[0352] Human cancer cells (NCI-H520, HARA, LK-2 and LUDLU 1) or normal human bronchial epithelial cells (HBEpC) were infected with vaccinia virus carrying hIL12 and hIL7 at an MOI of 1, or with vehicle (MOI 0). Cells were harvested at 6 h or 24 h post infection and the DNA amount of vaccinia virus carrying hIL12 and hIL7 was measured by standard quantitative polymerase chain reaction (qPCR) with primers designed to amplify the vaccinia virus hemagglutinin (HA) J7R Gene. Values are normalized to the 18s ribosomal RNA gene and presented as the mean of two replicate measurements.
[0353] like figure 2 shown that the amount of genomic DNA of hIL12 and hIL7-carrying vaccinia virus was detected in all hum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com