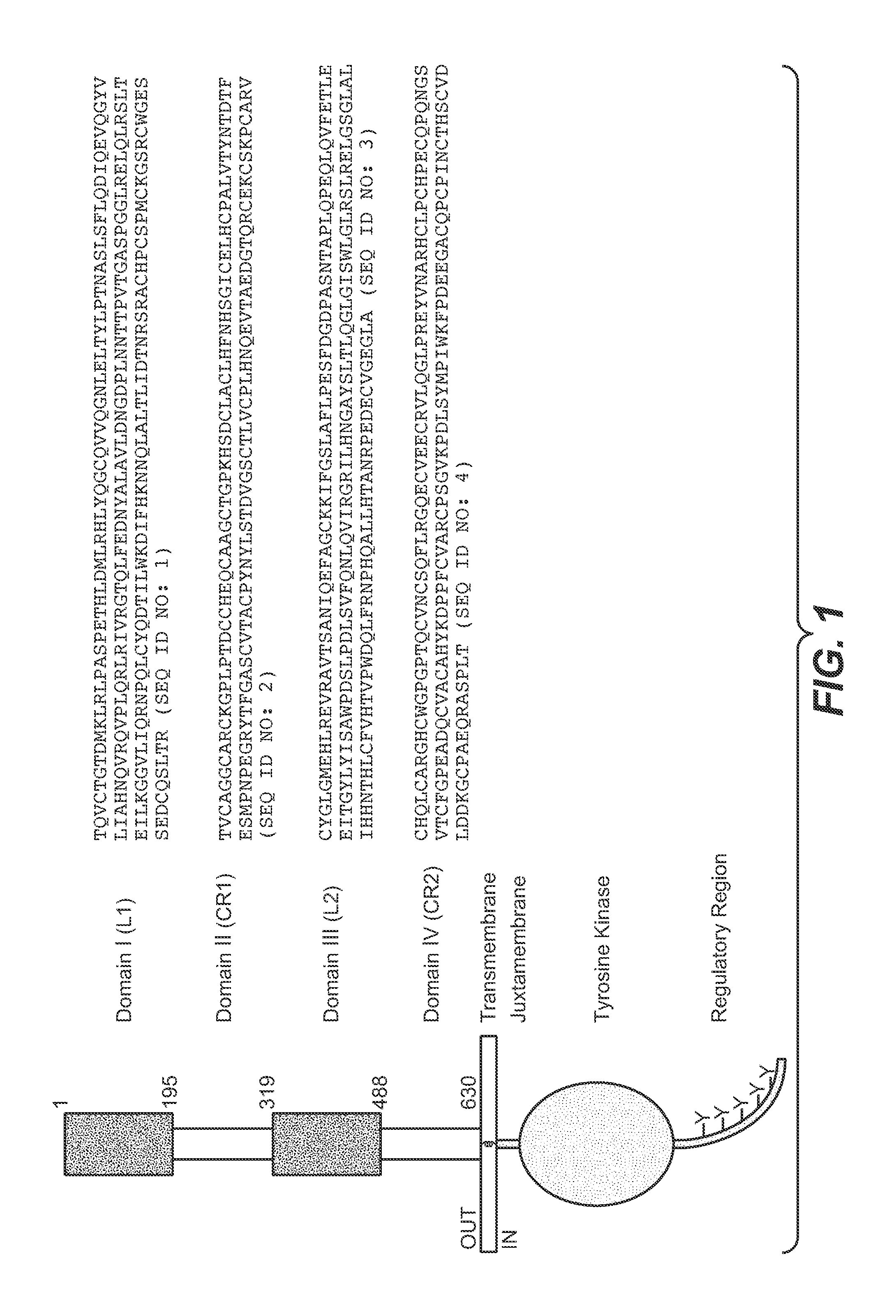
Uses for and article of manufacture including her2 dimerization inhibitor pertuzumab
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Phase IIa Study Evaluating Pertuzumab in Combination with Trastuzumab and Chemotherapy in Patients with HER2-Positive Advanced Gastric Cancer
[0254]Despite a sharp worldwide decline in incidence and a reduction in mortality during the second half of the twentieth century, gastric cancer remains the world's second leading cause of cancer mortality, after lung cancer (Parkin, D. Oncogene 23:6329-40 (2004)). The incidence of gastric cancer varies widely according to geographic region (Kelley et al. J Clin Epidemiol 56:1-9 (2003); Plummer et al. Epidemiology of gastric cancer. In: Butlet et al., editors. Mechanisms of carcinogenesis: contribution of molecular epidemiology. Lyon: IARC Scientific Publications No 157, IARC (2004)). In Japan, Korea, China, and certain countries in Central and South America, the incidence is 20 to 95 cases per 100,000 men. In contrast, in the United States, India, and Thailand, the incidence is 4 to 8 cases per 100,000 men. The incidence in Western Europe ran...
example 2
Phase III Study Evaluating Pertuzumab in Combination with Trastuzumab and Chemotherapy in Patients with HER2-Positive Advanced Gastric Cancer
[0366]This is a Phase III, randomized, open-label, multicenter clinical study designed to assess the efficacy of Pertuzumab in combination with Trastuzumab and chemotherapy in patients with HER2-positive locally advanced or metastatic gastric cancer.
[0367]Patients in the treatment arm receive Trastuzumab, cisplatin, and capecitabine and / or 5-fluorouracil. In the other arm, patients are given either placebo or Pertuzumab.
[0368]Treatment Regimens:
[0369]Pertuzumab:
[0370]840 mg dose for cycles 1-6.
[0371]Trastuzumab
[0372]8 mg / kg loading dose followed by 6 mg / kg q3w
[0373]Capecitabine
[0374]1000 mg / m2 bid d1-14 q3w×6
[0375]5-Fluorouracil
[0376]800 mg / m2 / day continuous iv infusion d1-5 q3w×6
[0377]Cisplatin
[0378]800 mg / m2 q3w×6
[0379]Primary End Point:
[0380]Overall survival (OS)
[0381]Secondary End Points:
[0382]Progression-free survival (PFS), time to diseas...
example 3
Results of a Phase III, Randomized, Double-Bind, Placebo-Controlled Registration Trial to Evaluate the Efficacy and Safety of Placebo+Trastuzumab+Docetaxel versus Pertuzumab+Trastuzumab+Docetaxel in Patients with Previously Untreated HER2-Positive Metastatic Breast Cancer (CLEOPATRA)
[0397]A protocol for evaluating Pertuzumab in HER2-positive metastatic breast cancer is found at http: / / clinicaltrials.gov / ct2 / show / NCT00567190 and in US 2009 / 0137387 as well as WO2009 / 154651.
[0398]This example concerns the clinical data obtained in the randomized, double-blind, placebo-controlled Phase III trial in patients with HER2-positive MBC, who had not received chemotherapy or biologic therapy for their metastatic disease. Patients were randomized 1:1 to receive placebo plus Trastuzumab plus Docetaxel or Pertuzumab plus Trastuzumab plus Docetaxel. The primary endpoint was progression-free survival (PFS), based on tumor assessments. PFS was defined as the time from randomization to the first docum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com