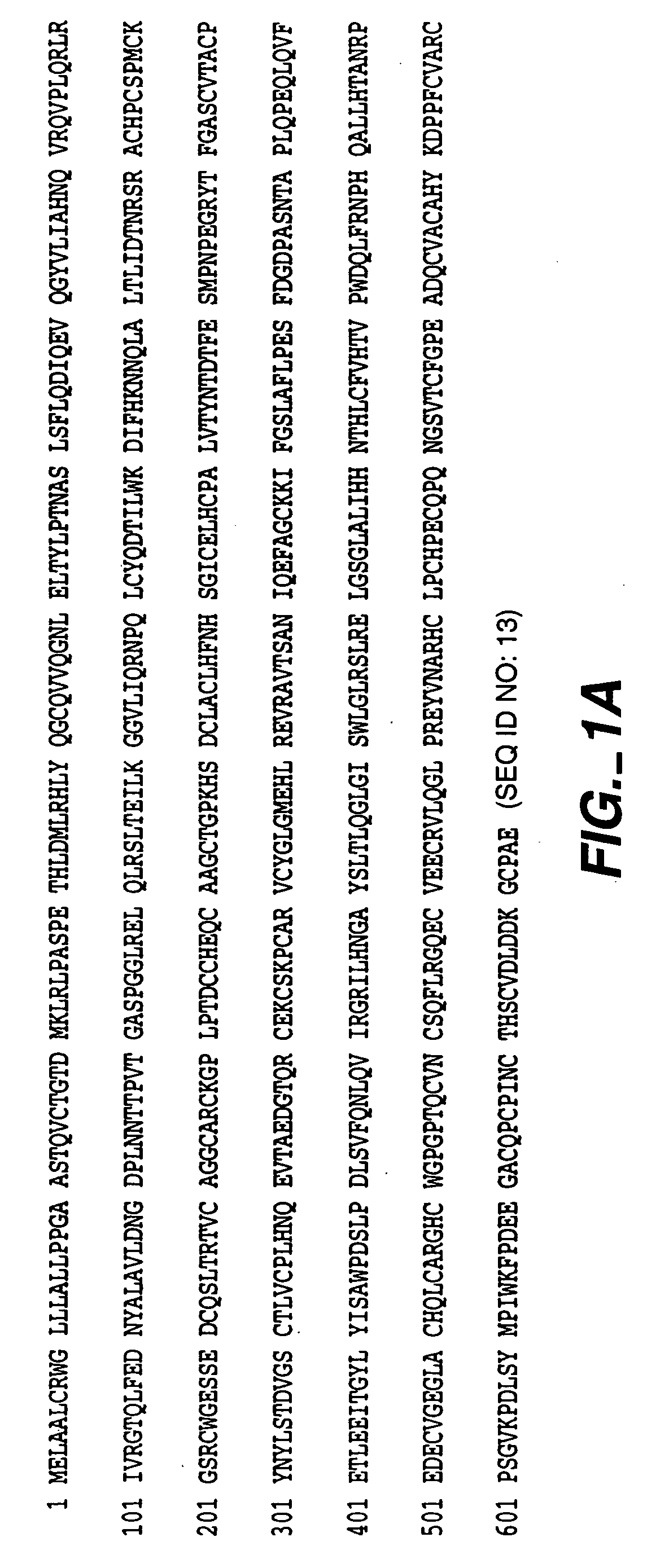
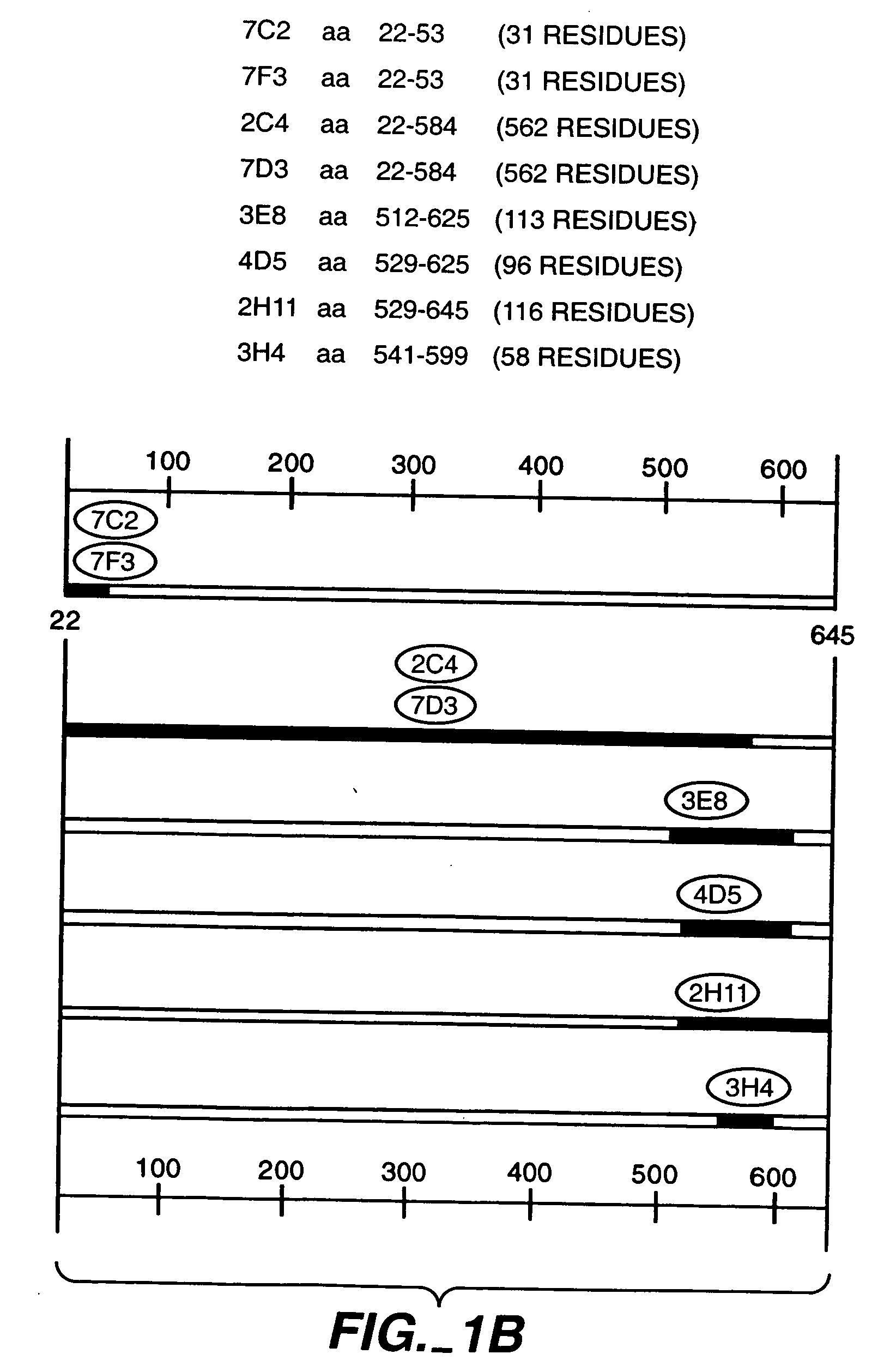
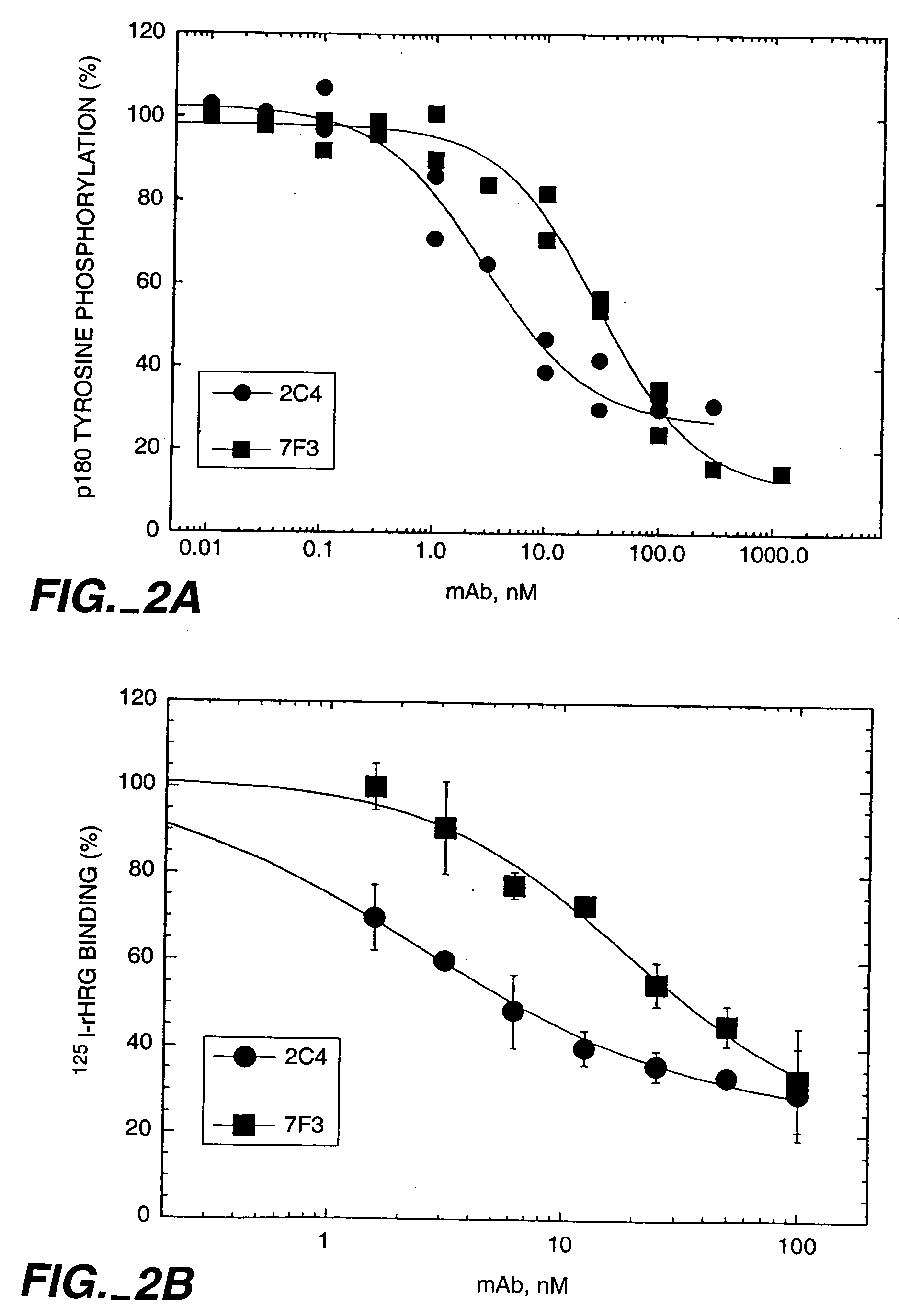
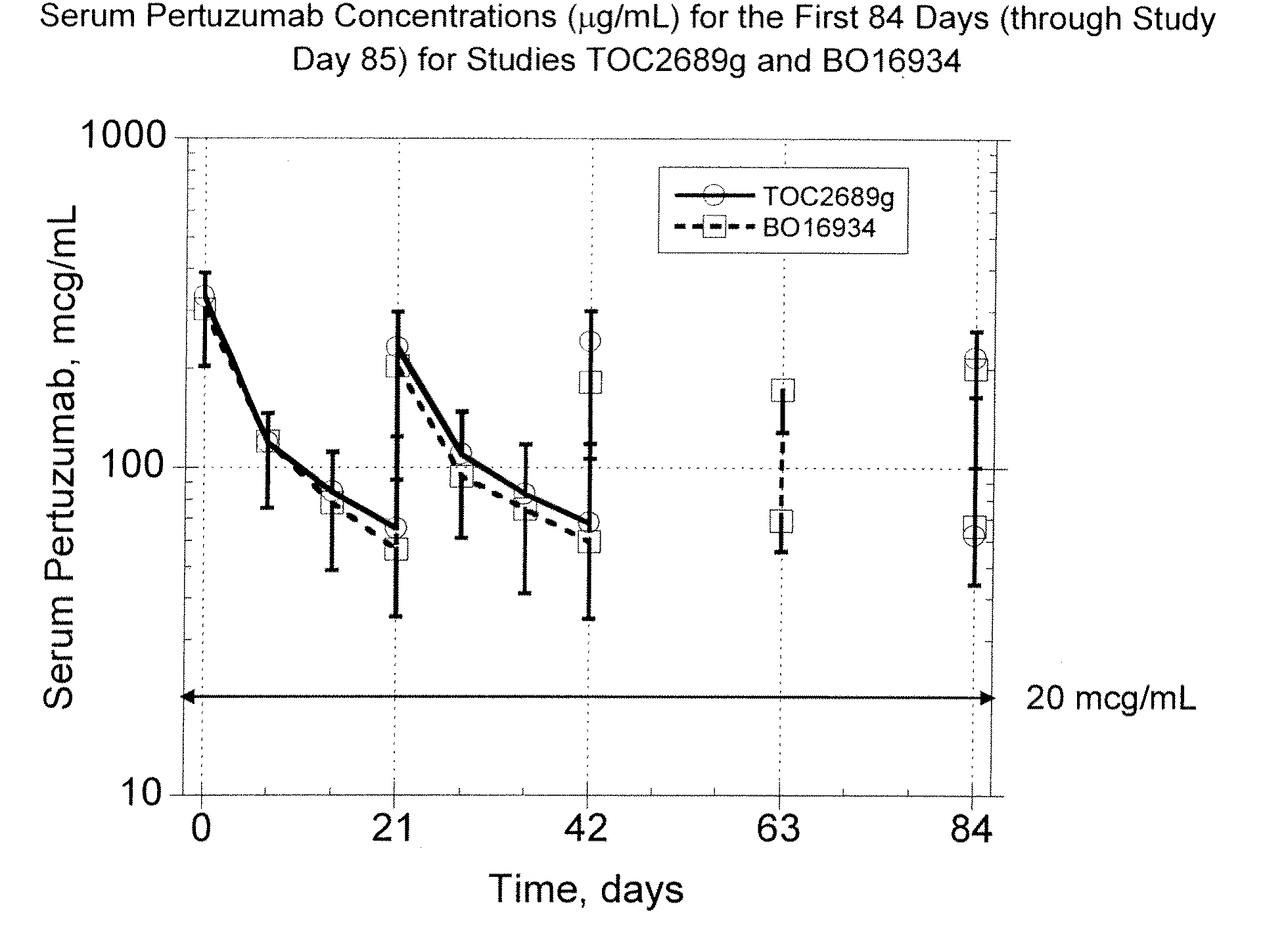
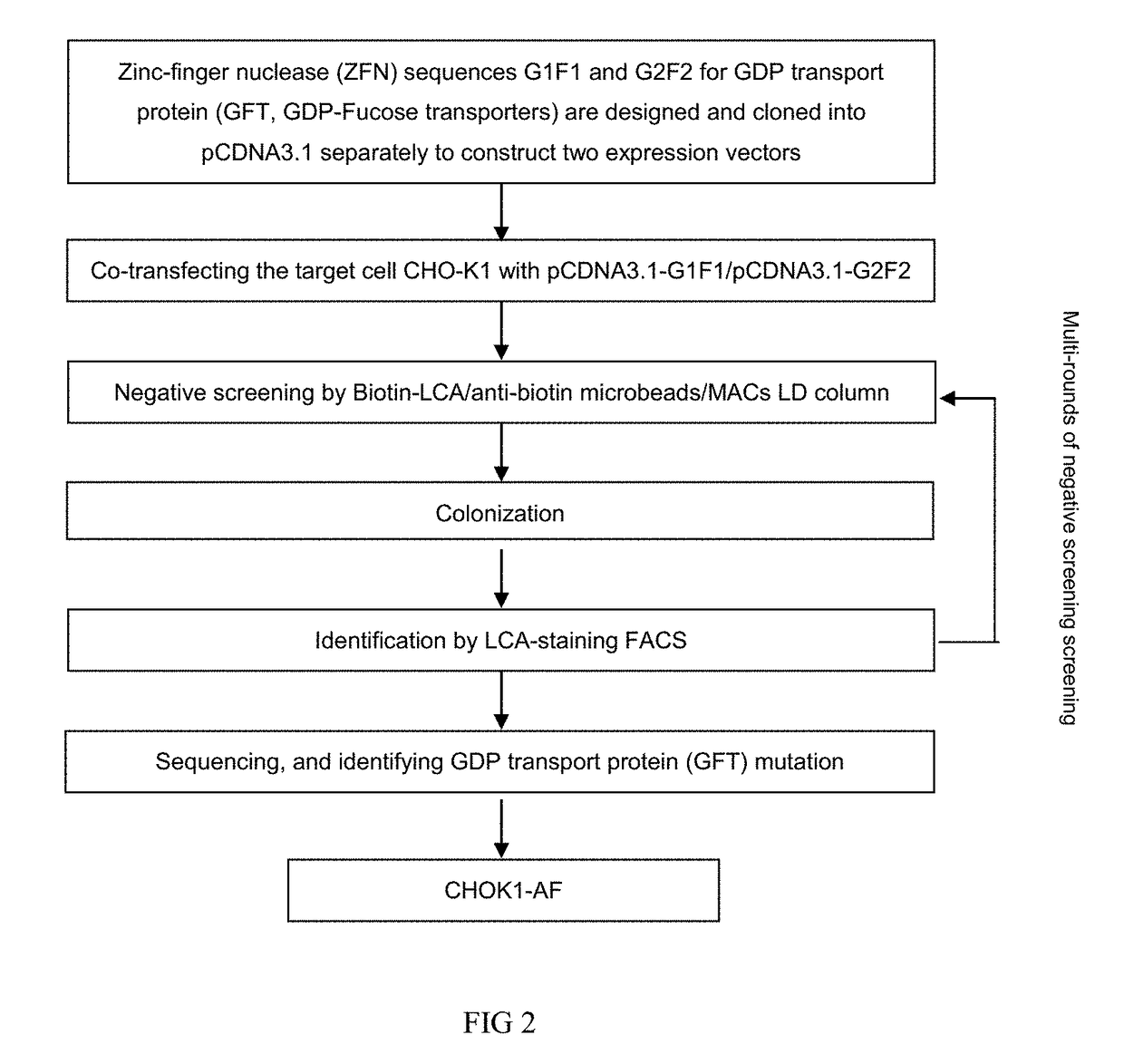
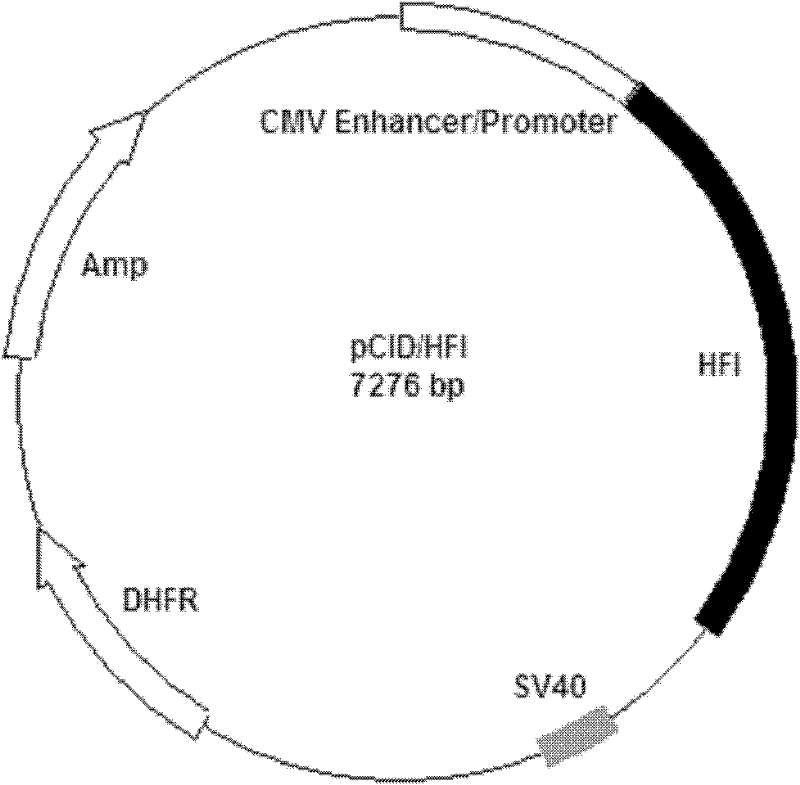
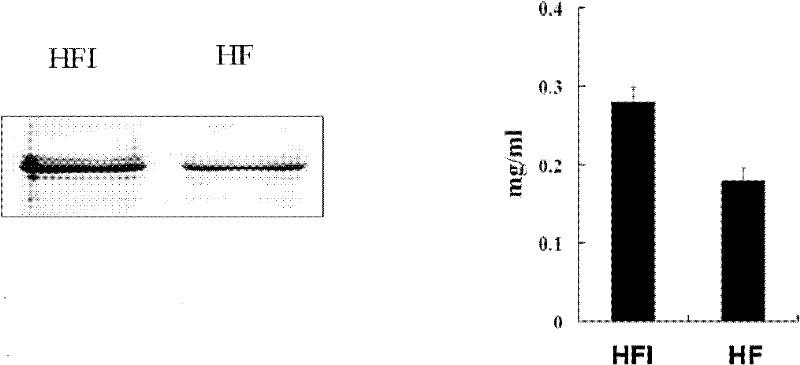
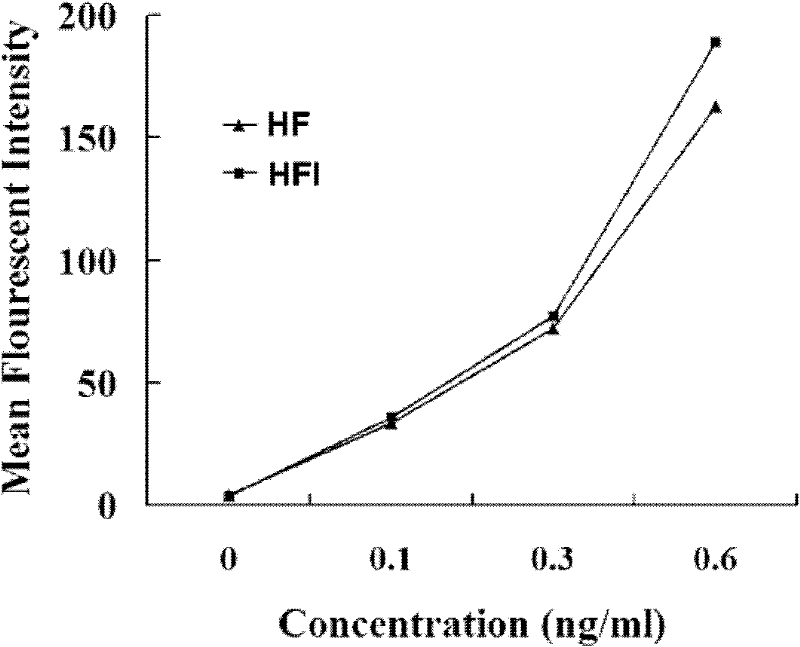
Patents
Literature
130 results about "HER2 Antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Therapy of platinum-resistant cancer
The present invention concerns a method for treating platinum-resistant, ovarian cancer, primary peritoneal carcinoma or fallopian tube carcinoma with the combination of a HER2 antibody that effectively inhibits HER dimerization as well as gemcitabine.
Owner:GENENTECH INC
Gene detection assay for improving the likelihood of an effective response to a her2 antibody cancer therapy
InactiveUS20070166753A1Great likelihoodChoose accuratelyOrganic active ingredientsBiocideAbnormal tissue growthAssay
The invention provides a method for more effective treatment of patients susceptible to or diagnosed with tumors overexpressing HER2, as determined by a gene amplification assay, with a HER2 antibody. Such method comprises administering a cancer-treating dose of the HER2 antibody, preferably in addition to chemotherapeutic agents, to a subject in whose tumor cells her2 has been found to be amplified e.g., by fluorescent in situ hybridization.
Owner:GENENTECH INC
Treatment of metastatic breast cancer
InactiveUS20090317387A1Improve survivalOrganic active ingredientsAntibody ingredientsBiologic therapiesDocetaxel-PNP
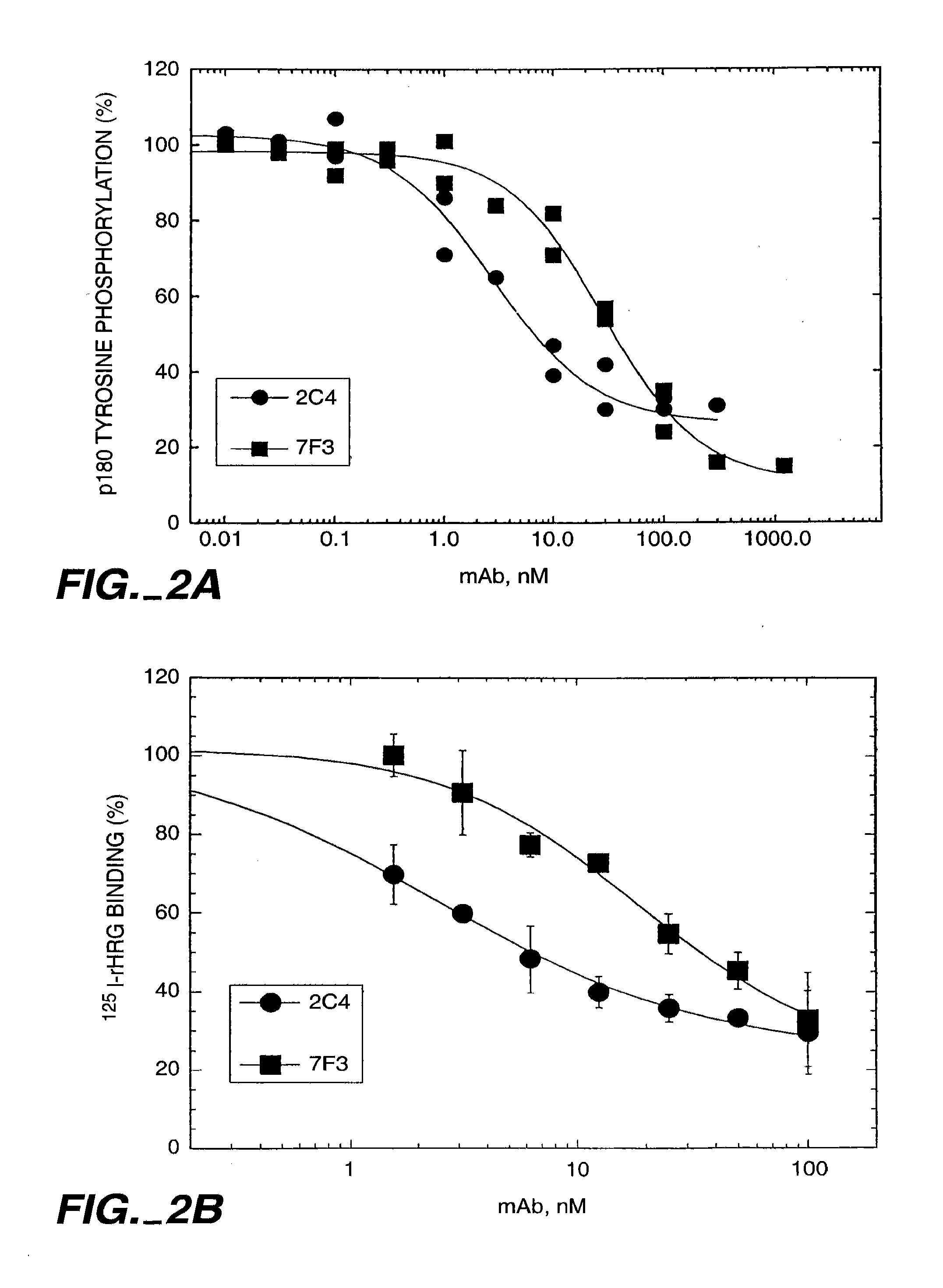
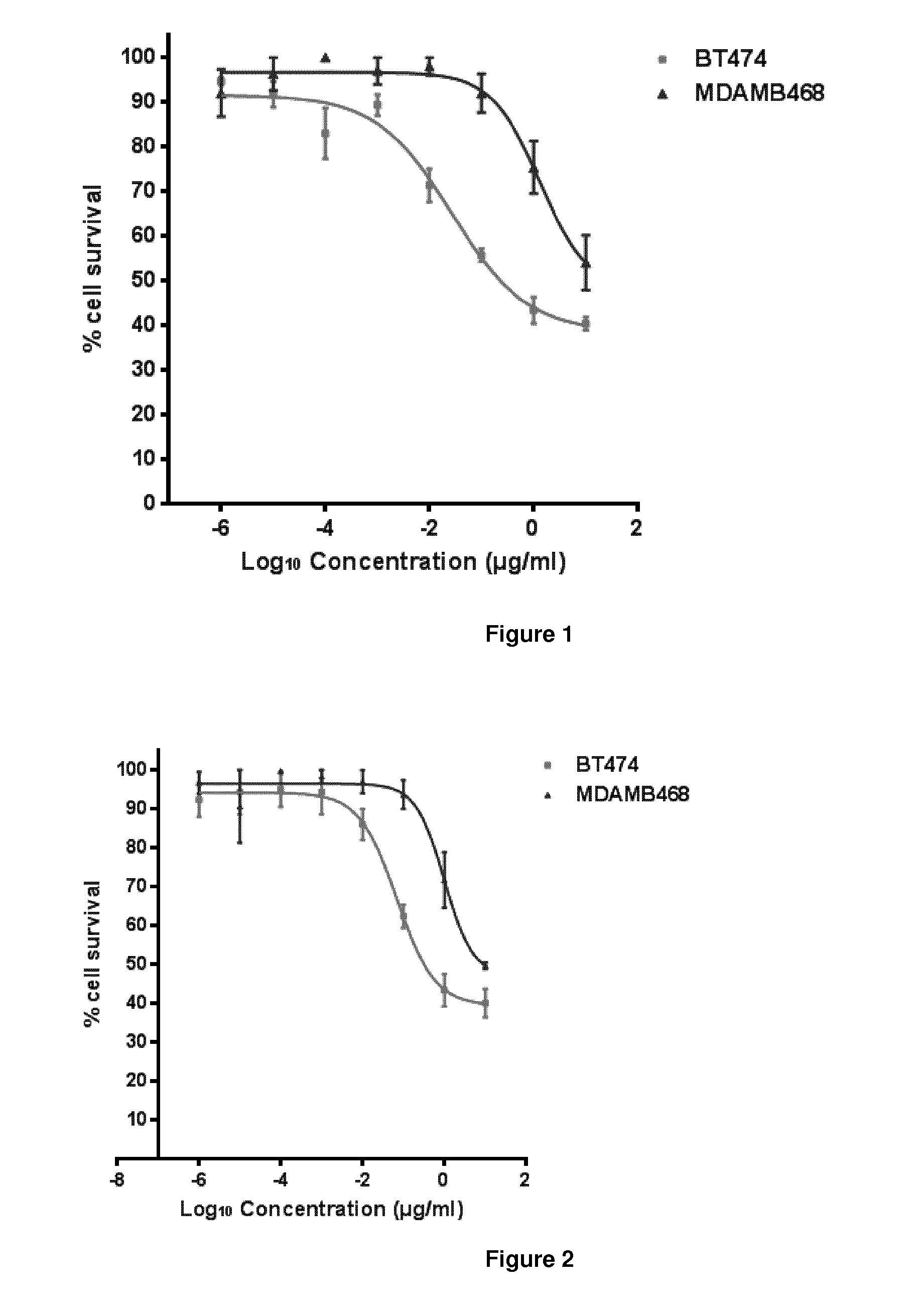
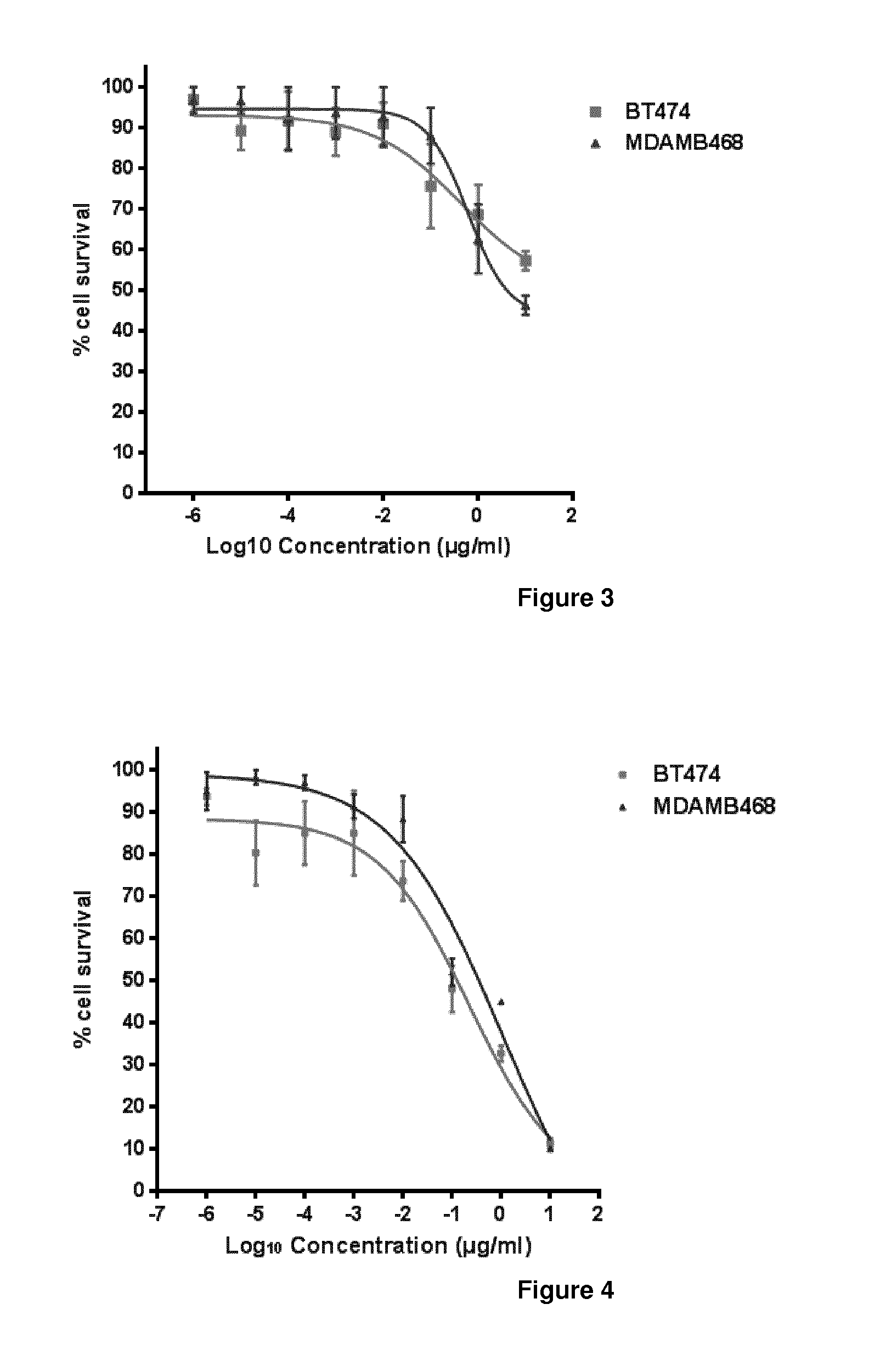
The present invention concerns treatment of previously untreated HER2-positive metastatic breast cancer with a combination of a growth inhibitory HER2 antibody, a HER2 dimerization inhibitor antibody and a taxane. In particular, the invention concerns the treatment of HER2-positive metastatic breast cancer in patients who did not receive prior chemotherapy or biologic therapy with a HER2 antibody binding essentially to epitope 2C4, a HER2 antibody binding essentially to epitope 4D5, and a taxane. The invention further comprises extending survival of such patients by the combination therapy of the present invention. In a preferred embodiment, the treatment involves administration of trastuzumab, pertuzumab and docetaxel.
Owner:GENENTECH INC
Tumor therapy with an Anti-vegf antibody
InactiveUS20080050385A1Prevents and reduces metastasisImmunoglobulins against growth factorsAntibody ingredientsAntiendomysial antibodiesTumor therapy
The present invention provides a method of treating a patient suffering from relapsed HER2 positive cancer with an anti-VEGF antibody during or after treatment with an anti-HER2 antibody. The invention also provides a kit comprising an anti-VEGF antibody.
Owner:F HOFFMANN LA ROCHE INC
Gene expression markers of tumor resistance to her2 inhibitor treatment
InactiveUS20100298156A1Inhibits HER ectodomain cleavageBlock ligand activationMicrobiological testing/measurementLibrary screeningWilms' tumorFhit gene
The present invention concerns markers of resistance of HER2 expressing tumors to treatment with HER2 inhibitors, such as HER2 antibodies, including trastuzumab.
Owner:GENENTECH INC
Methods of treating her2-positive cancers using pd-1 axis binding antagonists and Anti-her2 antibodies
InactiveUS20170008971A1Reduce functionIncreased activationOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesAnti antibody
The invention provides compositions and methods for treating HER2-postitive cancers. The method comprising administering a PD-1 axis binding antagonist and an antibody that targets HER2.
Owner:GENENTECH INC
Therapy of platinum-resistant cancer
The present invention concerns a method for treating platinum-resistant, ovarian cancer, primary peritoneal carcinoma or fallopian tube carcinoma with the combination of a HER2 antibody that effectively inhibits HER dimerization as well as gemcitabine.
Owner:GENENTECH INC
Pyrrolobenzodiazepine-Anti-her2 antibody conjugates
InactiveUS20150273077A1Organic active ingredientsAntibody ingredientsPyrrolobenzodiazepineHER2 Antibody
Owner:ADC THERAPEUTICS SA +1
Gene expression markers of tumor resistance to HER2 inhibitor treatment
ActiveUS20110151454A1Inhibition of activationNucleotide librariesMicrobiological testing/measurementNeoplasmTumor resistance
The present invention concerns markers of resistance of HER2 expressing tumors to treatment with HER2 inhibitors, such as HER2 antibodies, including trastuzumab.
Owner:GENENTECH INC
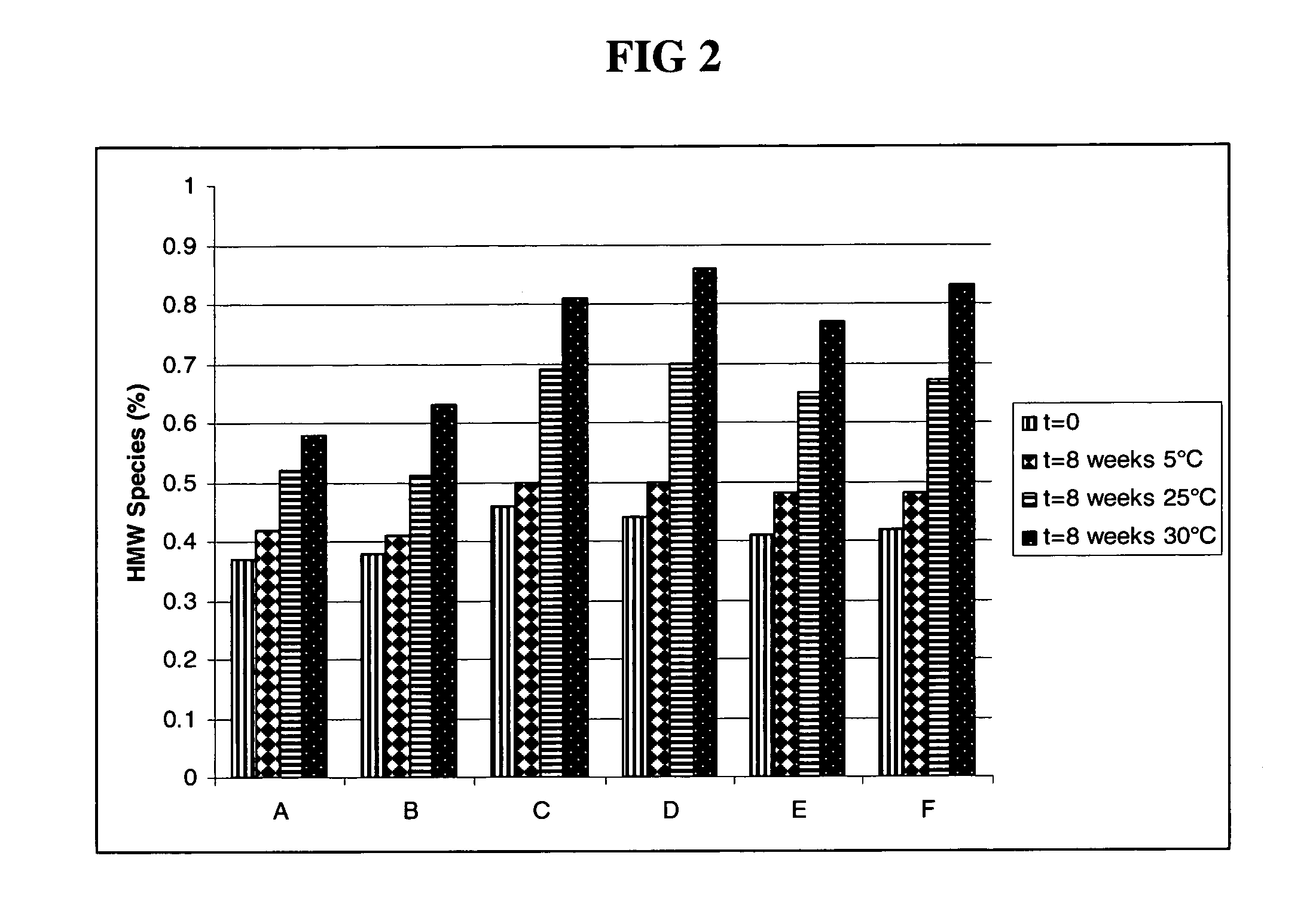
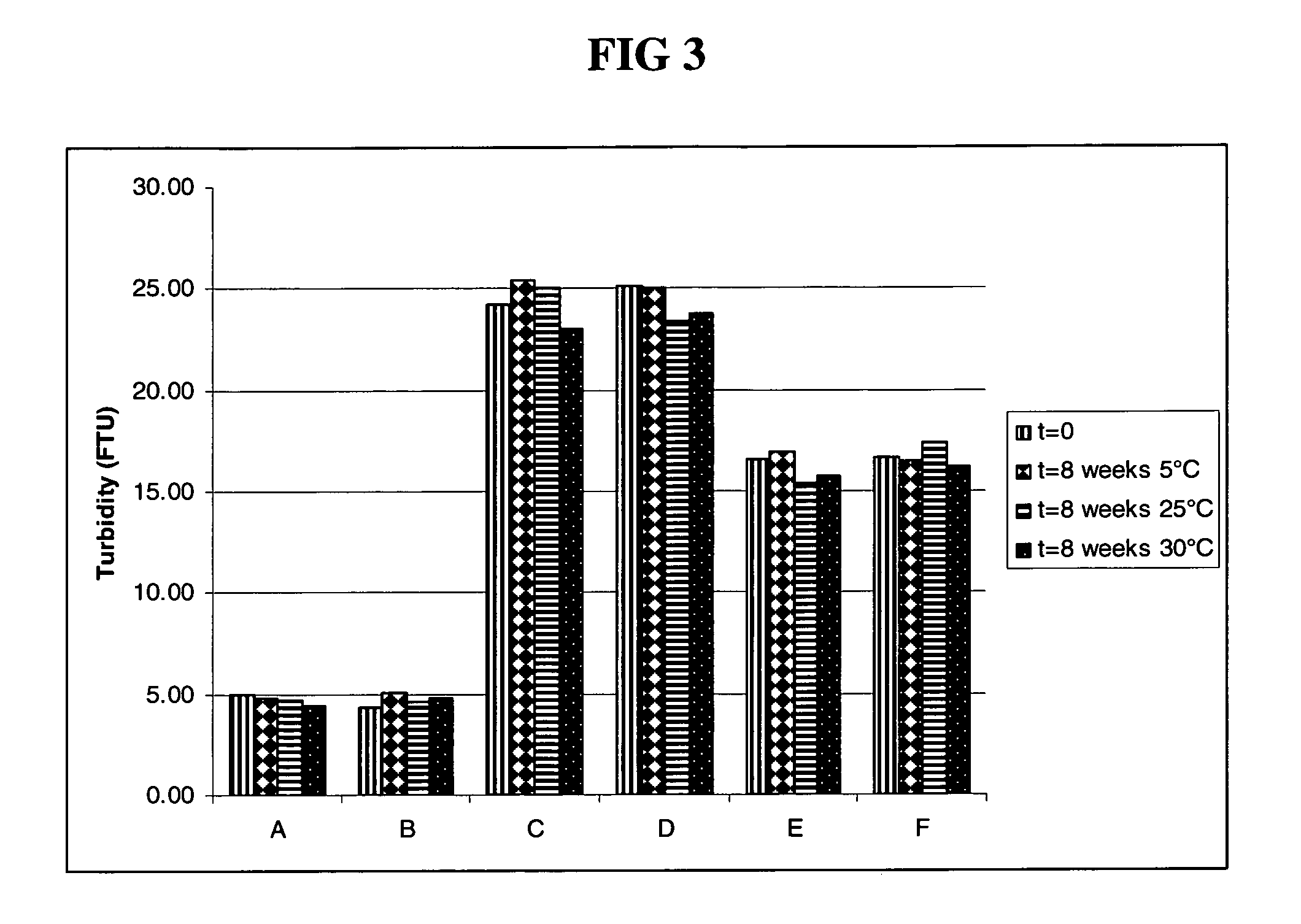
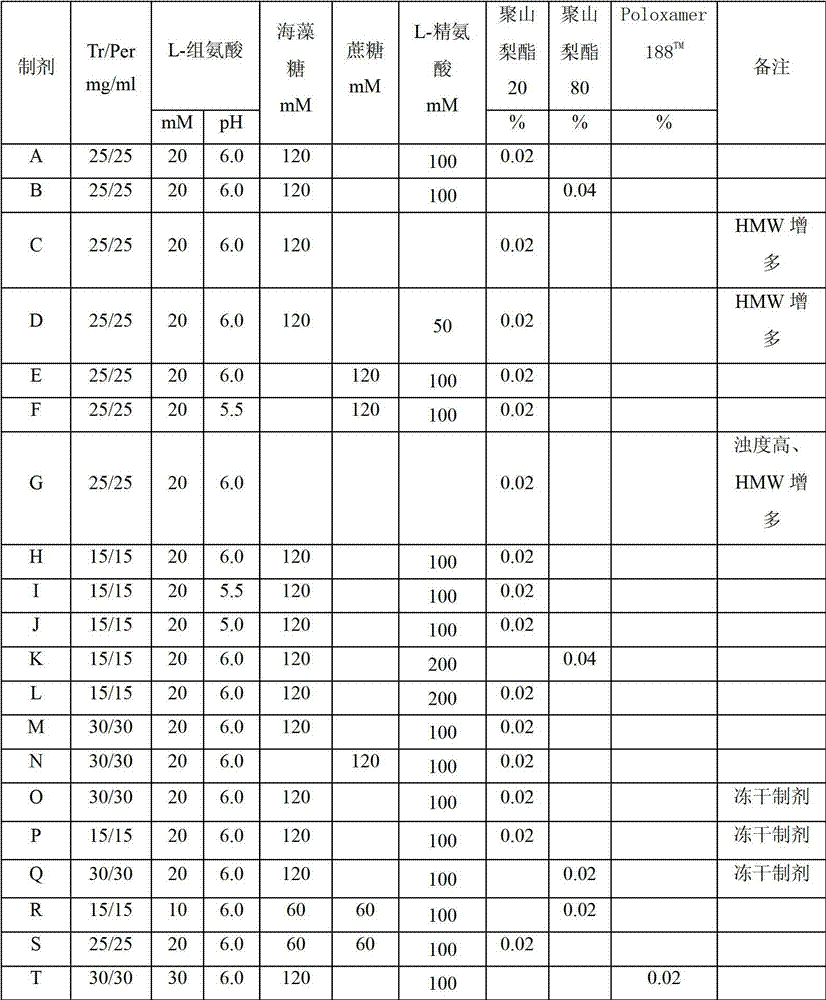
Subcutaneous anti-HER2 antibody formulations and uses thereof
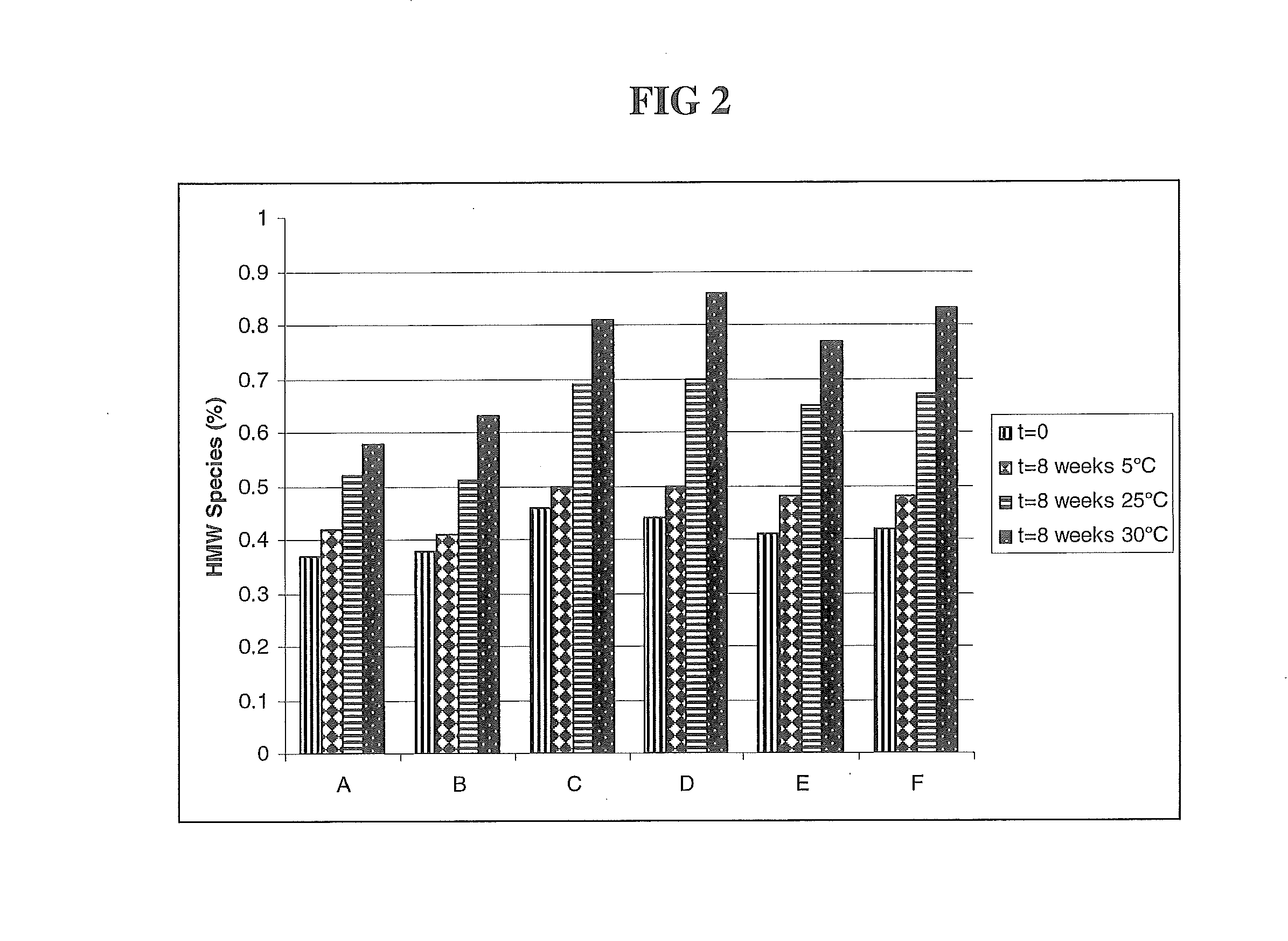
The present invention relates to a highly concentrated, stable pharmaceutical formulation of a pharmaceutically active anti-HER2 antibody, such as e.g. Trastuzumab (HERCEPTIN™), Pertuzumab or T-DM1, or a mixture of such antibody molecules for subcutaneous injection. In particular, the present invention relates to formulations comprising, in addition to a suitable amount of the anti-HER2 antibody, an effective amount of at least one hyaluronidase enzyme as a combined formulation or for use in form of a co-formulation. The formulations comprise additionally at least one buffering agent, such as e.g. a histidine buffer, a stabilizer or a mixture of two or more stabilizers (e.g. a saccharide, such as e.g. α,α-trehalose dihydrate or sucrose, and optionally methionine as a second stabilizer), a nonionic surfactant and an effective amount of at least one hyaluronidase enzyme. Methods for preparing such formulations and their uses thereof are also provided.
Owner:GENENTECH INC
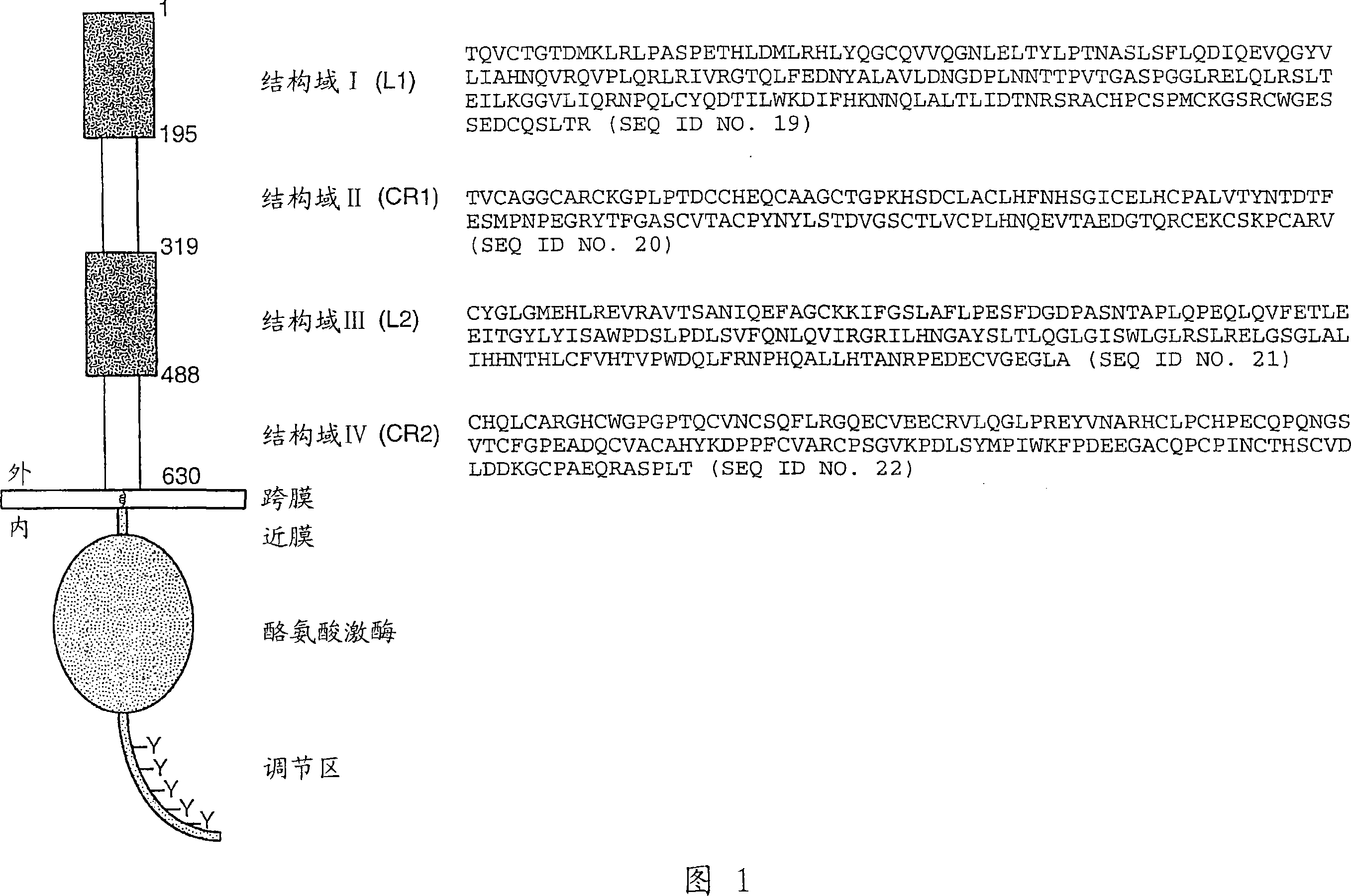
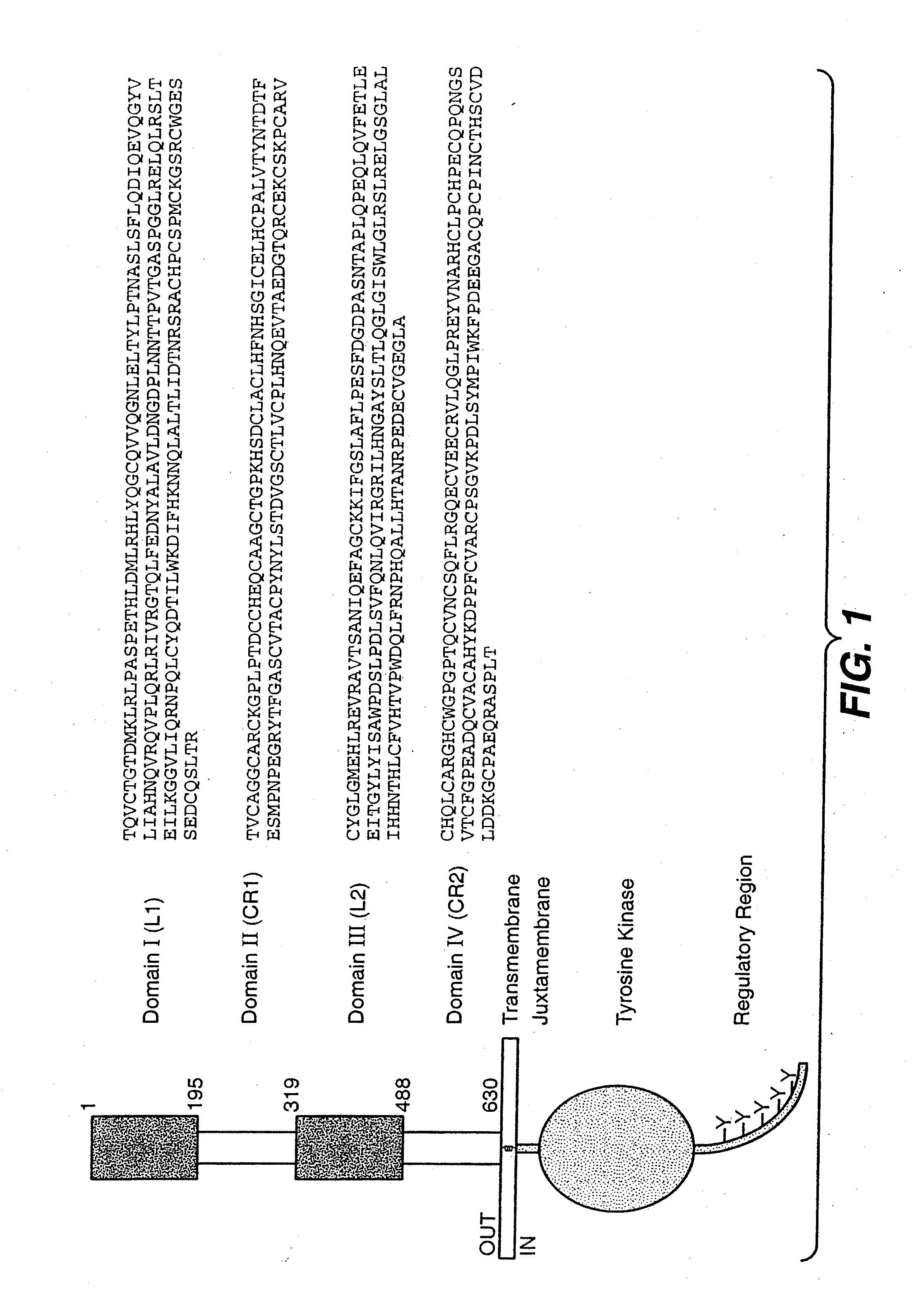
HER2 antibody composition
A composition comprising a main species HER2 antibody that binds to domain II of HER2, and an amino acid sequence variant thereof comprising an amino-terminal leader extension is disclosed. Pharmaceutical formulations comprising the composition, and therapeutic uses for the composition are also disclosed.
Owner:F HOFFMANN LA ROCHE & CO AG
Therapeutic Anti-her2 antibody fusion polypeptides
InactiveUS20090226466A1Enhanced cell killing of cellImprove efficiencySugar derivativesAntibody mimetics/scaffoldsTherapeutic proteinAnti her2
Therapeutic protein fusions comprising anti-HER2 antibody and MicB sequences are described along with methods for their production and use
Owner:GENENTECH INC
Antibody composition preparation and application thereof
ActiveCN102961745AAvoid formingAvoid gatheringAntibody ingredientsAntineoplastic agentsAnti her2Pertuzumab
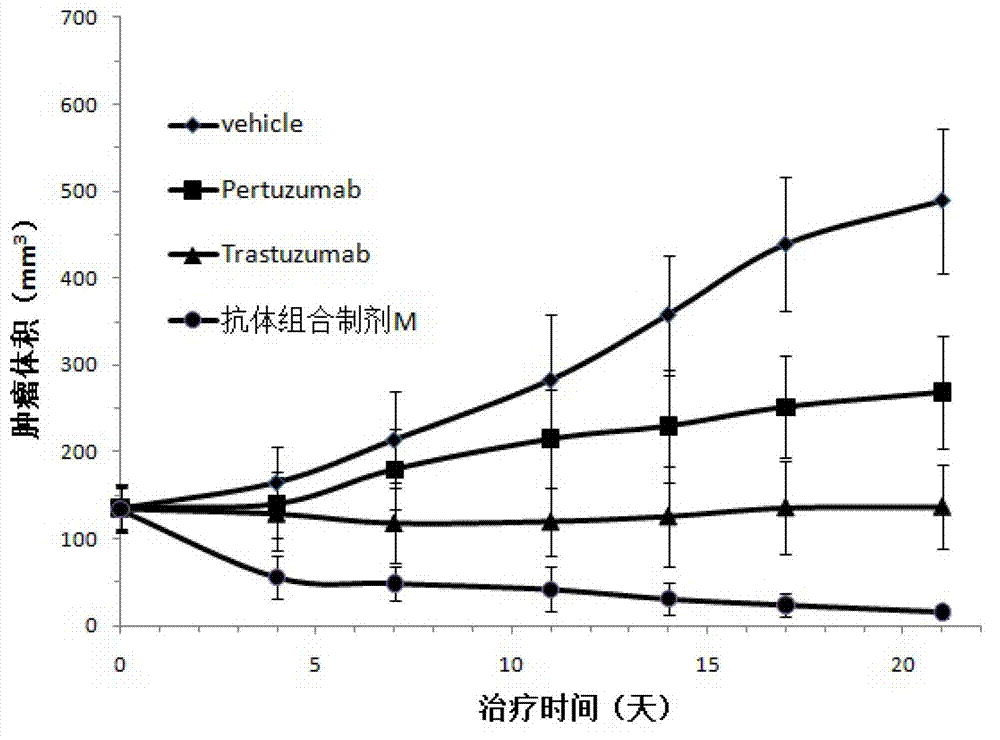
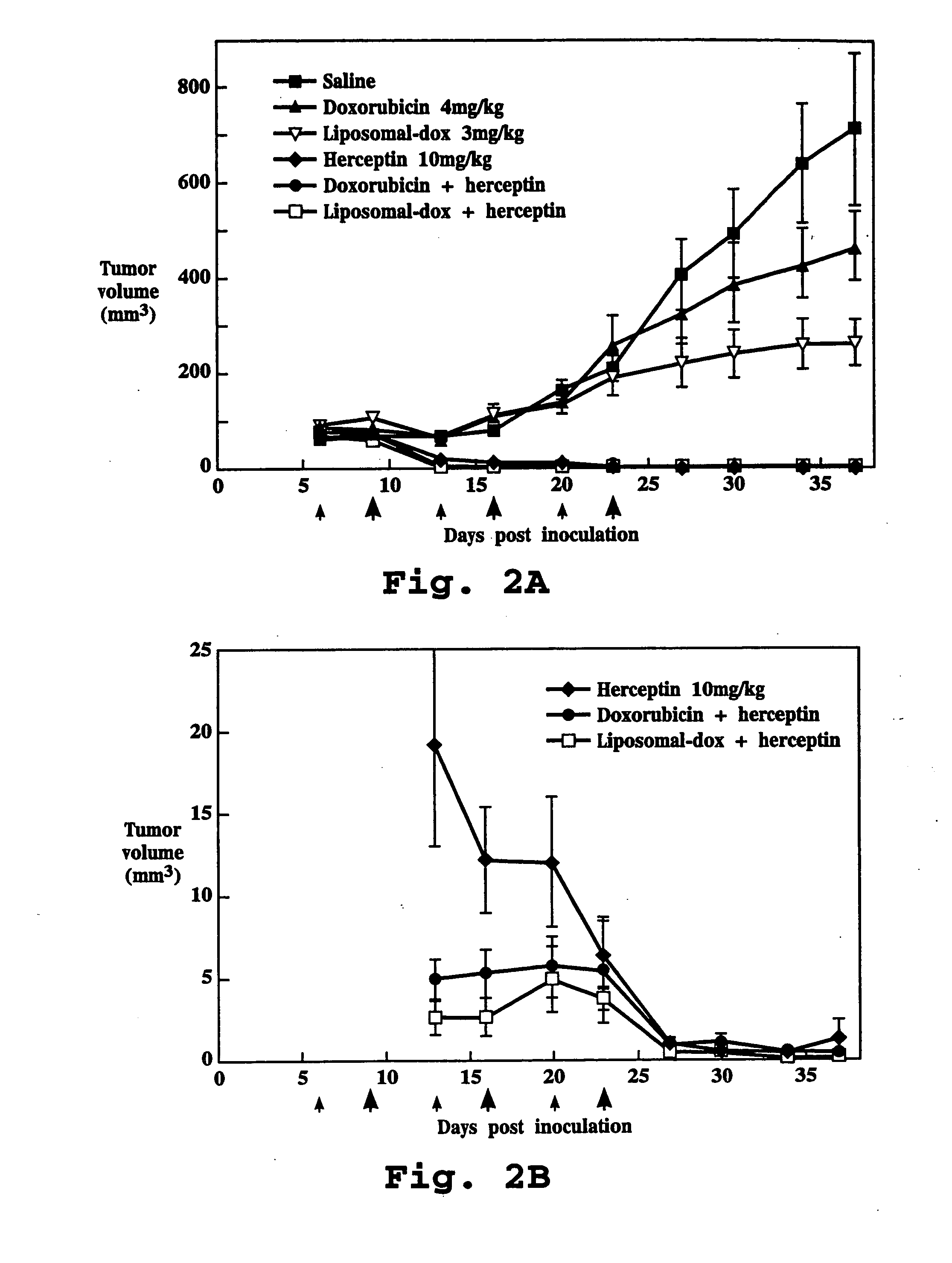
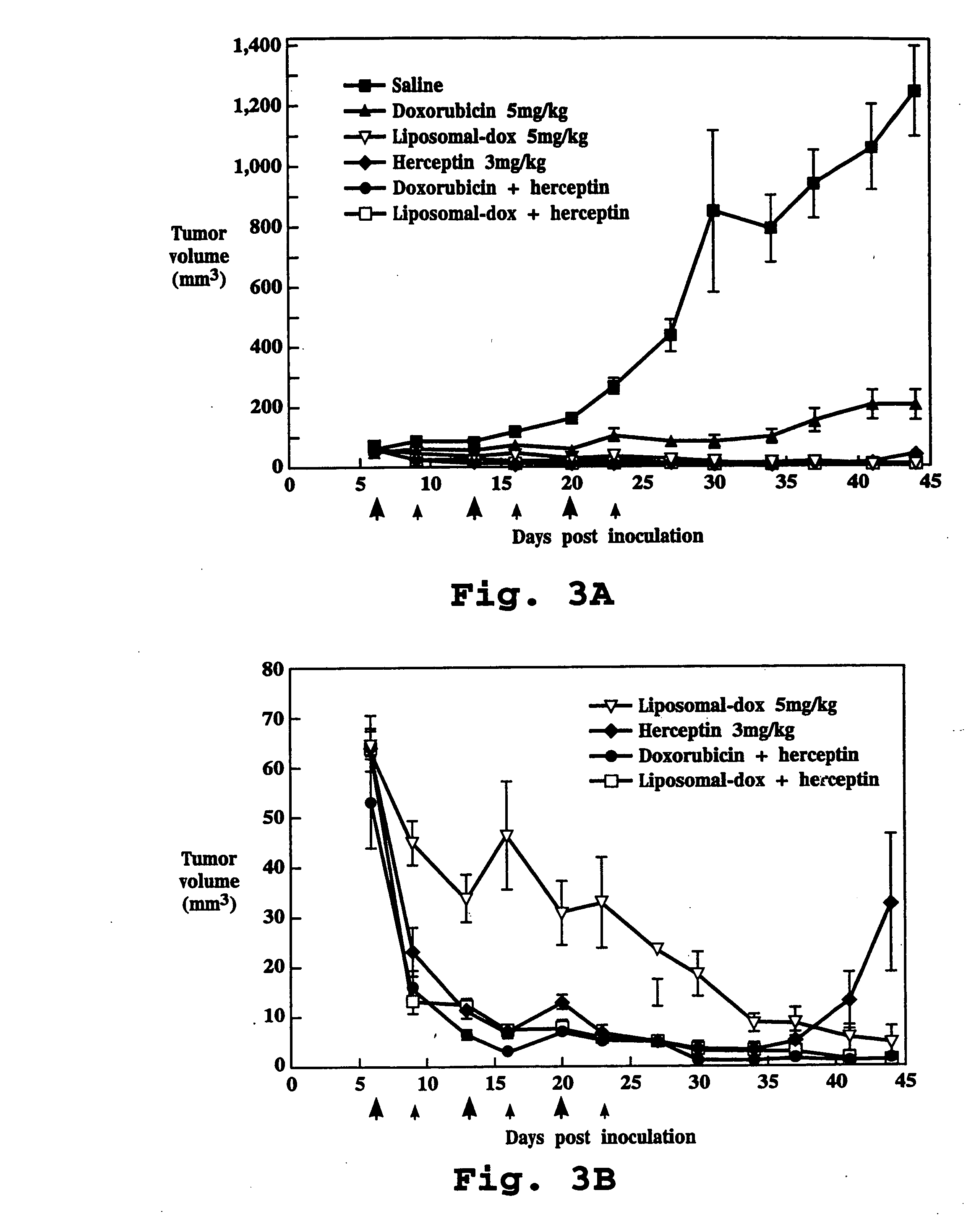
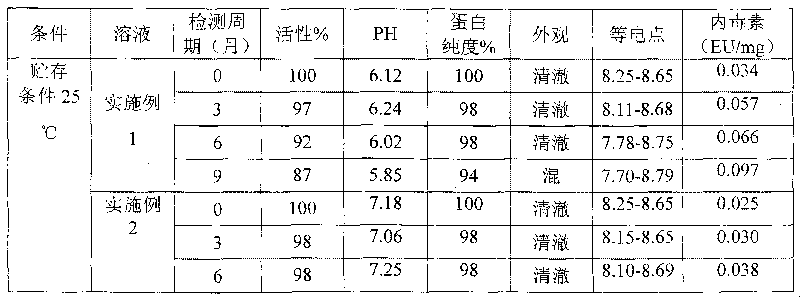
Relating to the technical field of pharmaceutical preparations, the invention provides an antibody composition preparation composed of Trastuzumab and Pertuzumab. The antibody composition preparation has better tumor treatment activity. Compared with the independent treatment of Trastuzumab and Pertuzumab, the composition preparation shows a synergistic effect of the two antibodies, and can reach a tumor control rate of 92.43%. And the preparation provided in the invention has better stability, thus being in favor of long-term preservation of the antibody composition preparation. The antibody composition preparation provided in the invention is applicable to preparation of drugs treating diseases or symptoms suited to anti-HER2 antibody therapy. During application, the antibody composition preparation disclosed in the invention is used with a chemotherapeutic agent jointly or sequentially.
Owner:SUZHOU KANGJU BIOTECHNOLOGY CO LTD +1
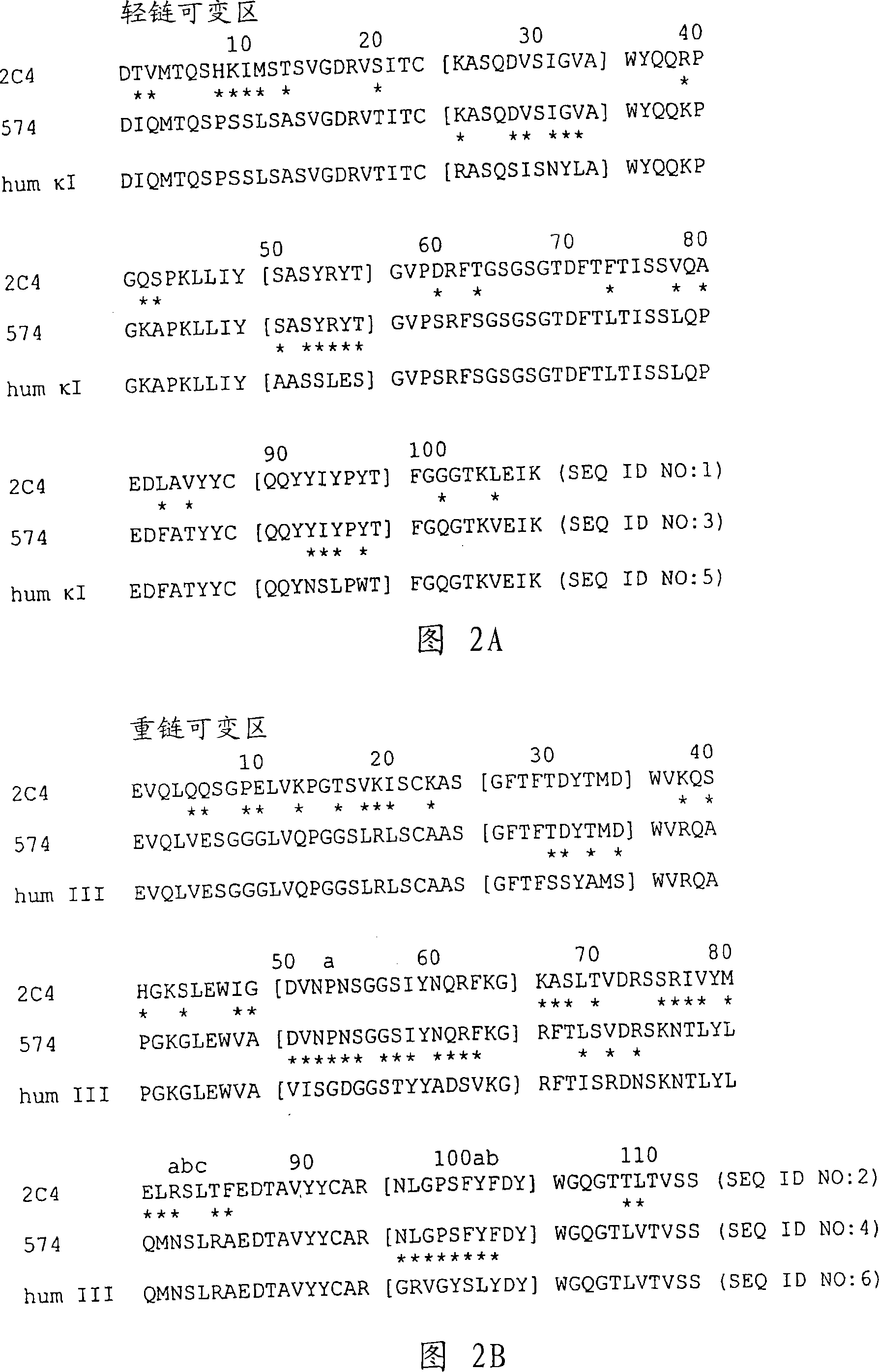
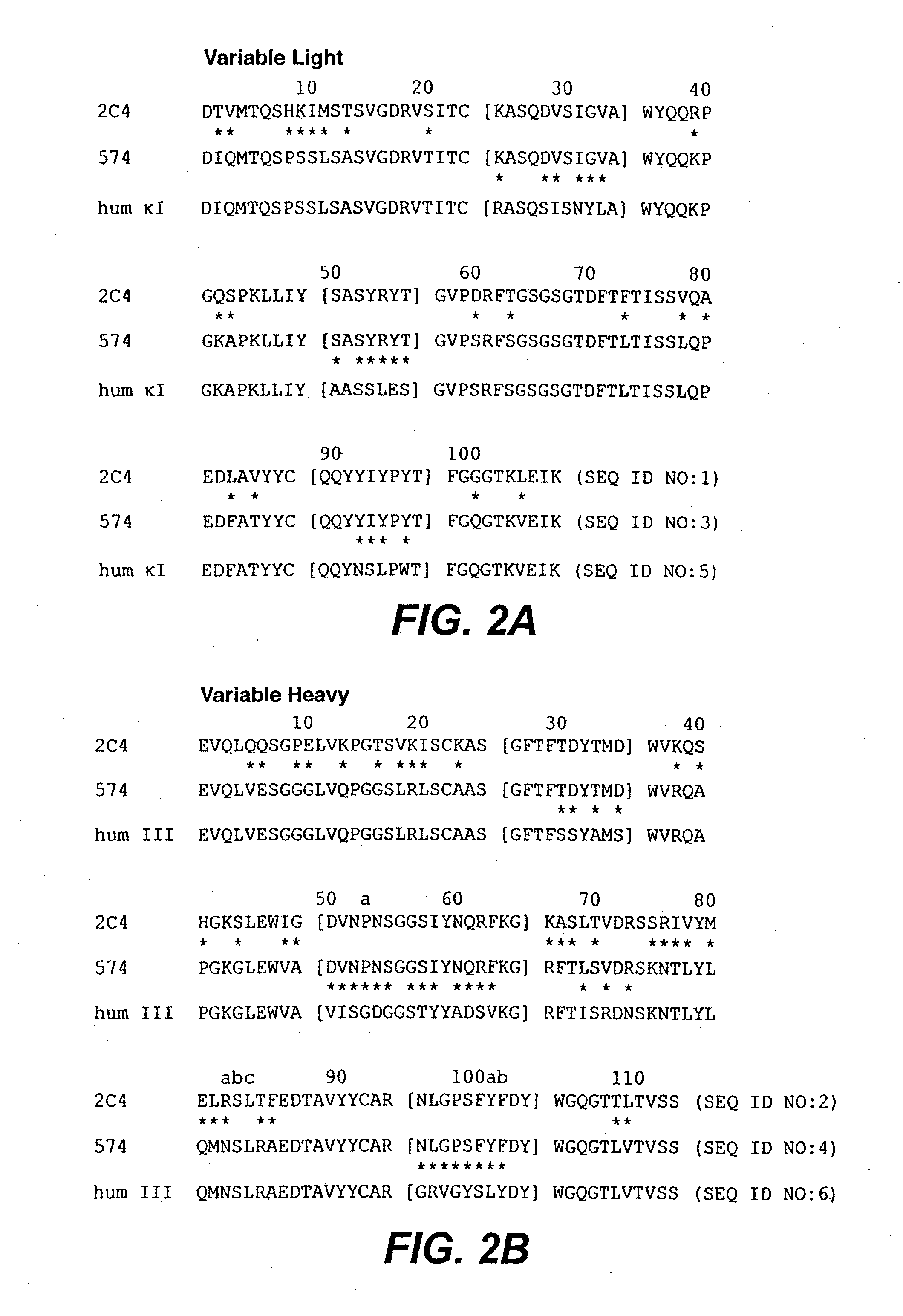
Anti-HER2 antibodies and compositions
The present invention relates to novel therapeutic antibodies directed against HER2 (ErbB2), as well as recombinant polyclonal anti-HER2 antibody compositions comprising at least two of said recombinant anti-HER2 antibodies, and use of the antibodies and antibody compositions for treatment of cancer.
Owner:SYMPHOGEN AS
Subcutaneous anti-HER2 Antibody Formulations and Uses Thereof
The present invention relates to a highly concentrated, stable pharmaceutical formulation of a pharmaceutically active anti-HER2 antibody, such as e.g. Trastuzumab (HERCEPTIN™), Pertuzumab or T-DM1, or a mixture of such antibody molecules for subcutaneous injection. In particular, the present invention relates to formulations comprising, in addition to a suitable amount of the anti-HER2 antibody, an effective amount of at least one hyaluronidase enzyme as a combined formulation or for use in form of a co-formulation. The formulations comprise additionally at least one buffering agent, such as e.g. a histidine buffer, a stabilizer or a mixture of two or more stabilizers (e.g. a saccharide, such as e.g. α,α-trehalose dihydrate or sucrose, and optionally methionine as a second stabilizer), a nonionic surfactant and an effective amount of at least one hyaluronidase enzyme. Methods for preparing such formulations and their uses thereof are also provided.
Owner:GENENTECH INC
Treatment of metastatic breast cancer
InactiveUS20140044704A1Improve survivalOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDocetaxel-PNPDocetaxel
The present invention concerns treatment of previously untreated HER2-positive metastatic breast cancer with a combination of a growth inhibitory HER2 antibody, a HER2 dimerization inhibitor antibody and a taxane. In particular, the invention concerns the treatment of HER2-positive metastatic breast cancer in patients who did not receive prior chemotherapy or biologic therapy with a HER2 antibody binding essentially to epitope 2C4, a HER2 antibody binding essentially to epitope 4D5, and a taxane. The invention further comprises extending survival of such patients by the combination therapy of the present invention. In a preferred embodiment, the treatment involves administration of trastuzumab, pertuzumab and docetaxel.
Owner:GENENTECH INC
Method for potentiating activity of a chemotherapeutic drug
InactiveUS20050084524A1High activityIncreased toxicityBiocideHeavy metal active ingredientsAntiendomysial antibodiesReceptor
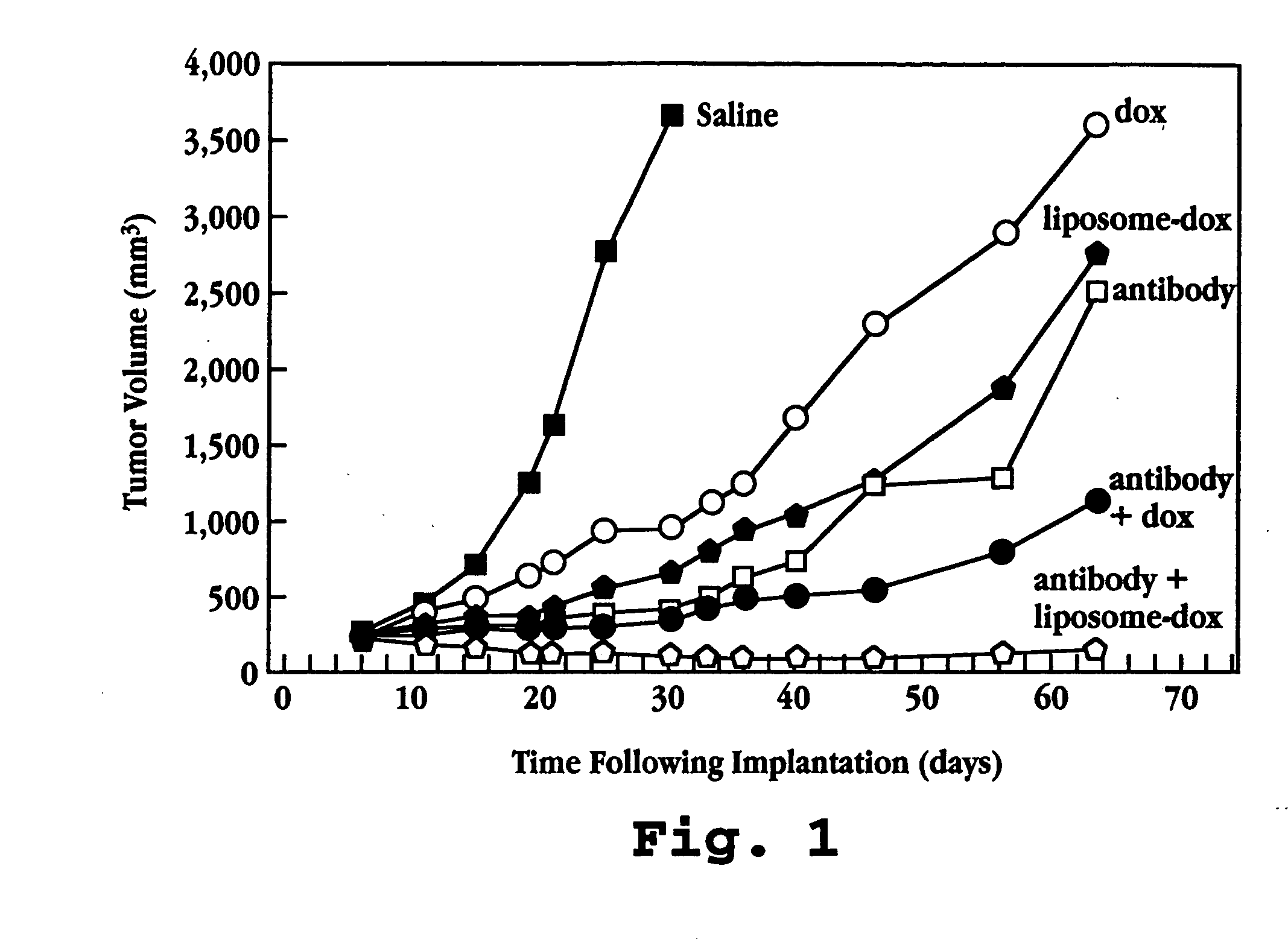
A method for potentiating the activity of a chemotherapeutic drug administered in combination with a biological agent is described. The method includes entrapping the chemotherapeutic drug in a liposome and administering the liposome-entrapped drug in combination with the biological agent. The method is particularly useful for treatment of cancer which over-express tyrosine kinase receptor and for B-cell lymphomas, where, for example, anti-HER2 antibodies or anti-CD19 antibodies are administered in combination with the cytotoxic drug.
Owner:ALZA CORP
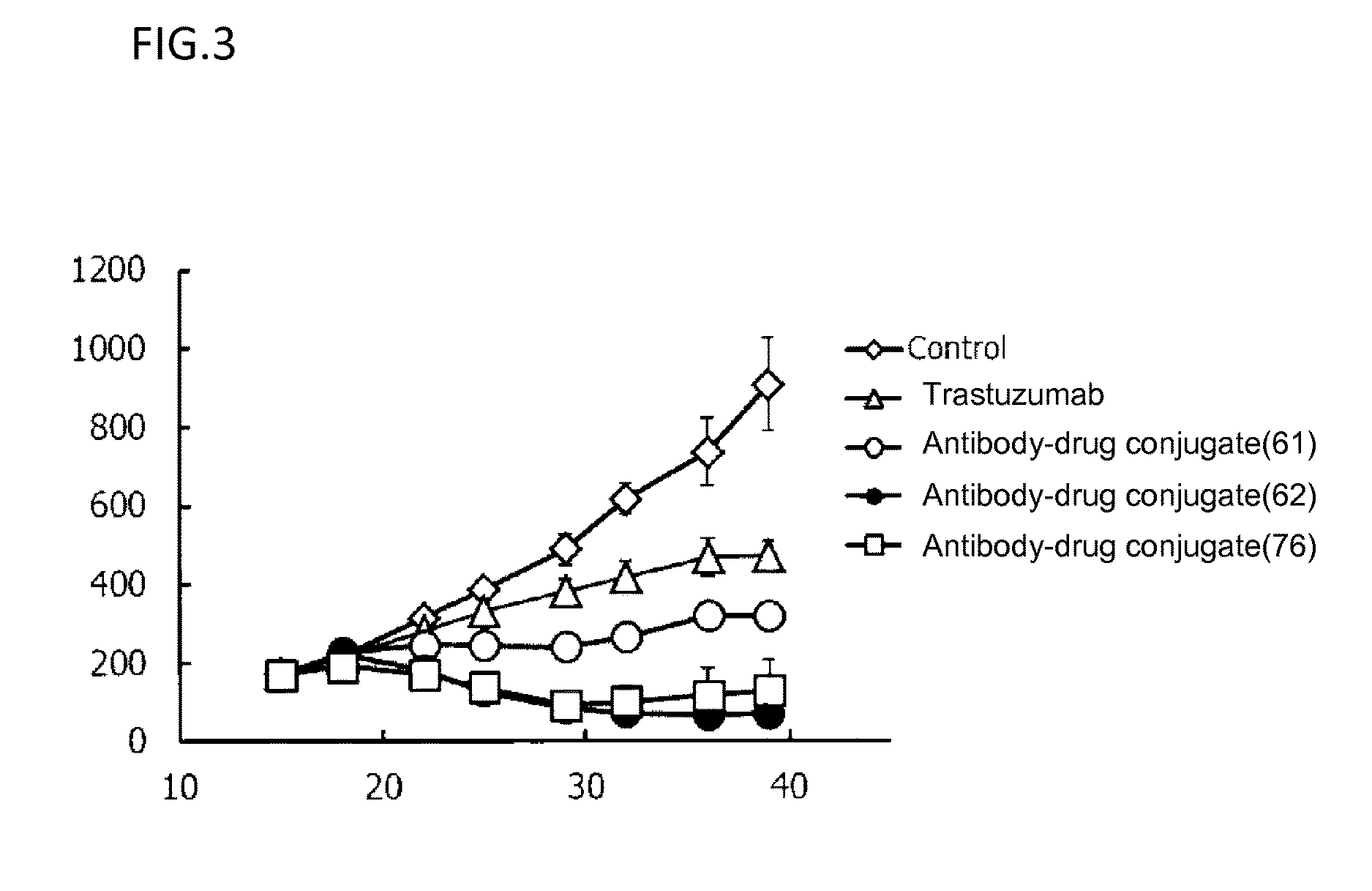
(Anti-her2 antibody)-drug conjugate
ActiveUS20170035906A1Good antitumor effectImprove securityOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDrug conjugationAntiendomysial antibodies
As an antitumor drug which is excellent in terms of antitumor effect and safety and has an excellent therapeutic effect, there is provided an antibody-drug conjugate in which an antitumor compound represented by the following formula is conjugated to an anti-HER2 antibody via a linker having a structure represented by the formula: L1-L2-LP-NH—(CH2)n1-La-Lb-Lc or -L1-L2-LP- wherein the anti-HER2 antibody is connected to the terminal L1, and the antitumor compound is connected to the terminal Lc or LP with the nitrogen atom of the amino group at position 1 as the connecting position.
Owner:DAIICHI SANKYO CO LTD
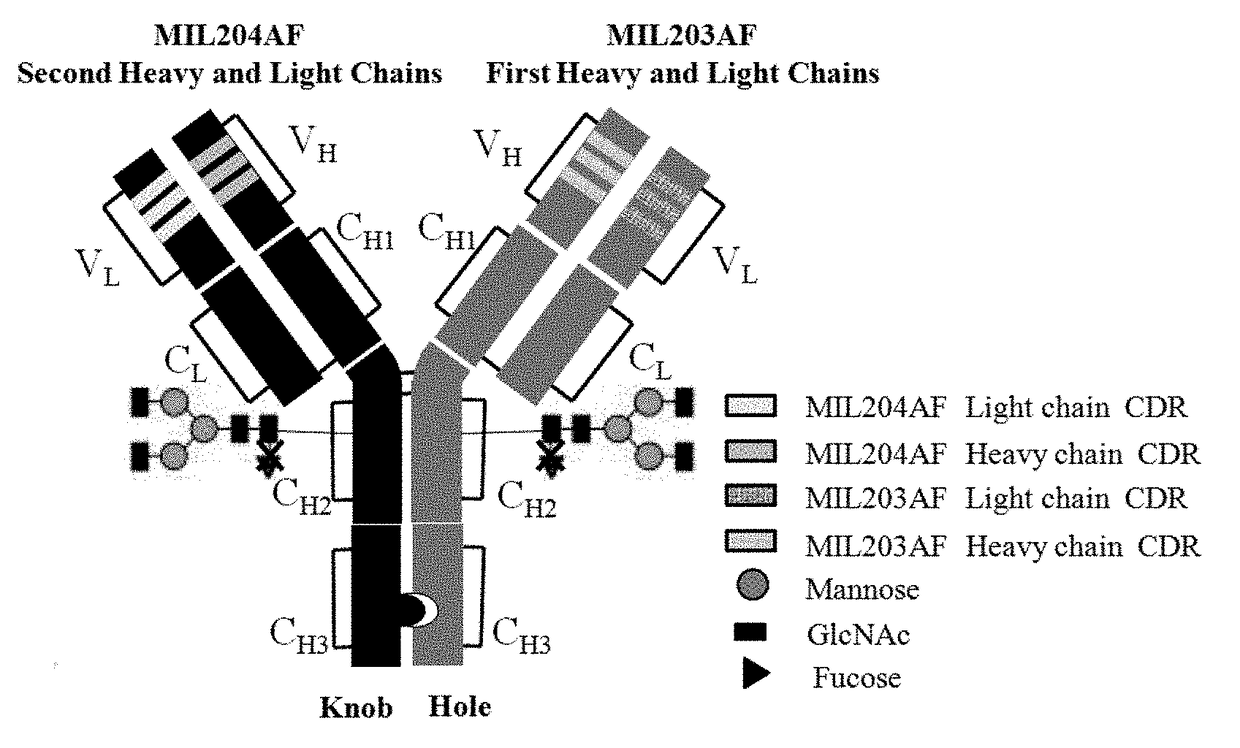
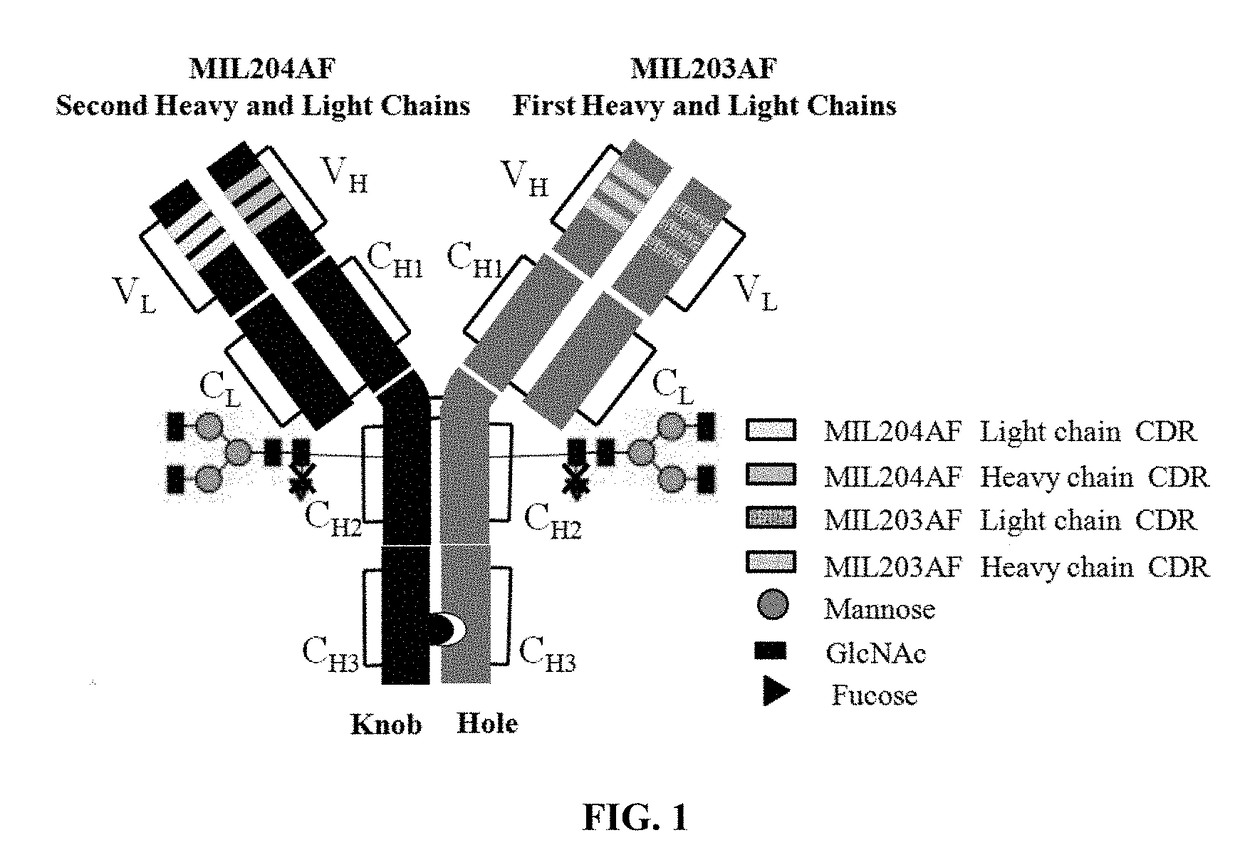
Bispecific Anti-her2 antibody
The present invention relates to humanized bispecific anti-HER2 antibodies that comprise one antigen binding site containing variable regions of heavy and light chain of trastuzumab, and another antigen binding site containing variable regions of heavy and light chain of pertuzumab. The bispecific anti-HER2 antibodies is effective for treating cancer, such as breast cancer, gastric cancer, or ovarian cancer. Preferred bispecific anti-HER antibodies of the present invention are afucosylated antibodies. The present invention also relates to Chinese Hamster ovary (CHO) mutant cell line that has a dysfunctional Slc35C1 gene, which is the only dysfunctional gene in the mutant that affects glycan regulation.
Owner:BEIJING MABWORKS BIOTECH
Fusion protein of Her2 antibody and interleukin 2 and application thereof
InactiveCN102199218AImprove biological activityIncrease productionFungiBacteriaAntiendomysial antibodiesCD16
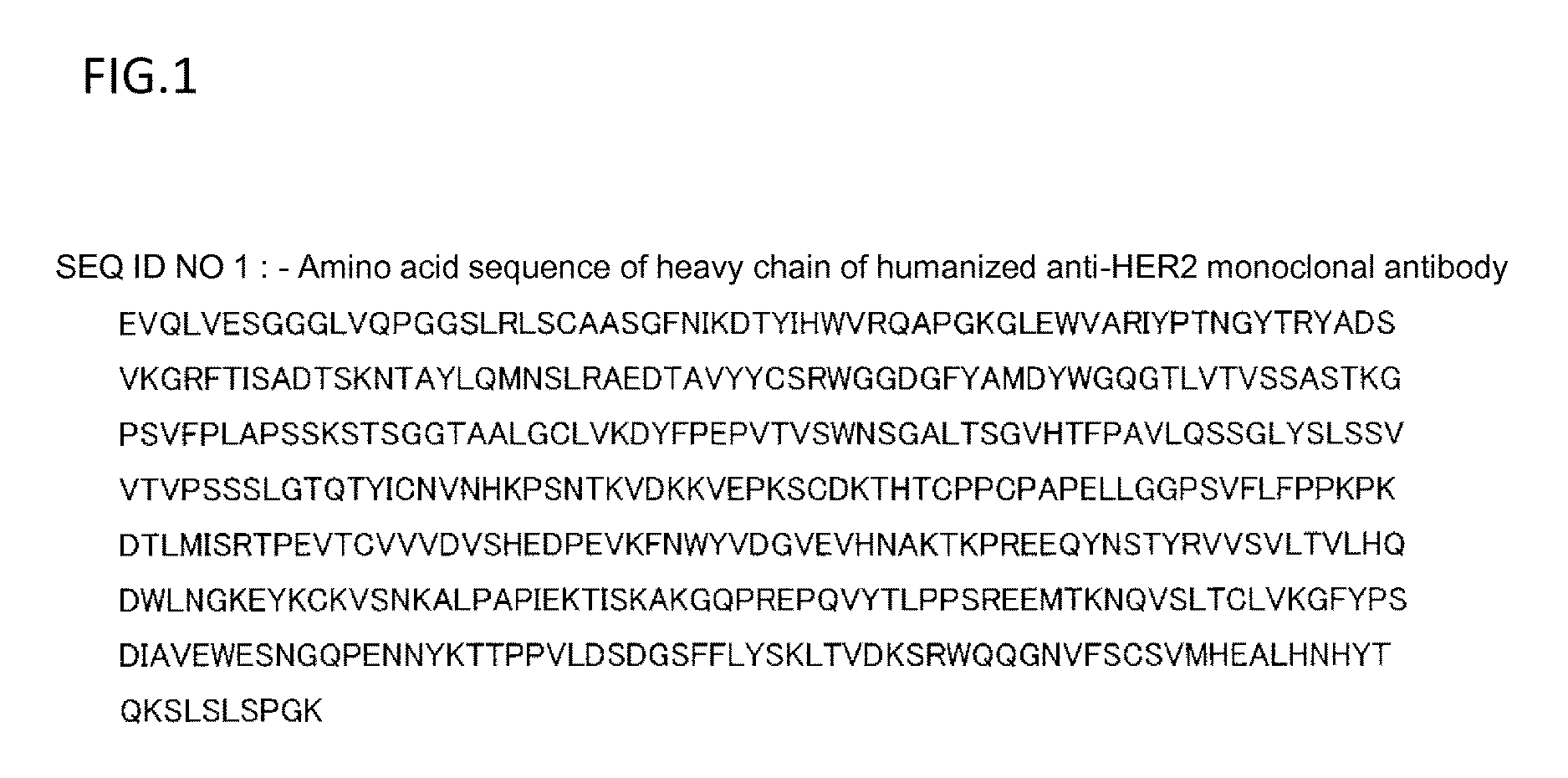
The invention discloses a fusion protein of Her2 antibody and interleukin 2 and application thereof, and belongs to the technical fields of medical science and oncology phymatology. The fusion protein comprises CD16 monoclonal antibody heavy chain signal peptide, ErbB2 antibody heavy chain variable region, a Linker 1, a light chain variable region, a human antibody Fc segment, a Linker 2 and IL-2 mature peptide, and has the amino acid sequence shown as SEQ ID No.1. The fusion protein can inhibit Herceptin resistant breast cancer cell proliferation, and can kill Her2 high-expression breast cancer cells. The invention lays a foundation for clinical application of the fusion protein of the Her2 antibody and the interleukin 2 in the anti-tumor aspect.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
HER2 antibody composition
ActiveCN101023100BAntibody ingredientsImmunoglobulinsAntiendomysial antibodiesPharmaceutical formulation
A composition comprising a main species HER2 antibody that binds to domain II of HER2, and an amino acid sequence variant thereof comprising an amino-terminal leader extension is disclosed. Pharmaceutical formulations comprising the composition, and therapeutic uses for the composition are also disclosed.
Owner:F HOFFMANN LA ROCHE & CO AG
Subcutaneous her2 antibody formulations
ActiveUS20180296470A1Good dispersionOrganic active ingredientsPeptide/protein ingredientsCancer therapyPertuzumab
Fixed dose HER2 antibody formulations for subcutaneous administration are provided along with their use in the treatment of cancer. The formulations include fixed dose subcutaneous formulations of pertuzumab and subcutaneous co-formulations of pertuzumab and trastuzumab, and their use in the treatment of cancer.
Owner:F HOFFMANN LA ROCHE & CO AG
Anti-tumor bispecific miniaturized antibody with double functions of targeting therapy and detection
ActiveCN103951754ASignificant effectQuick clearImmunoglobulins against cell receptors/antigens/surface-determinantsMacromolecular non-active ingredientsADAMTS ProteinsBio engineering
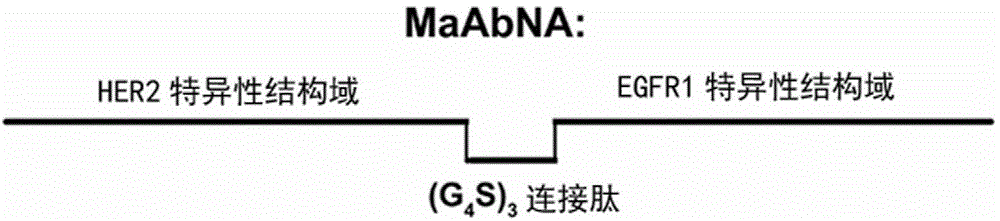
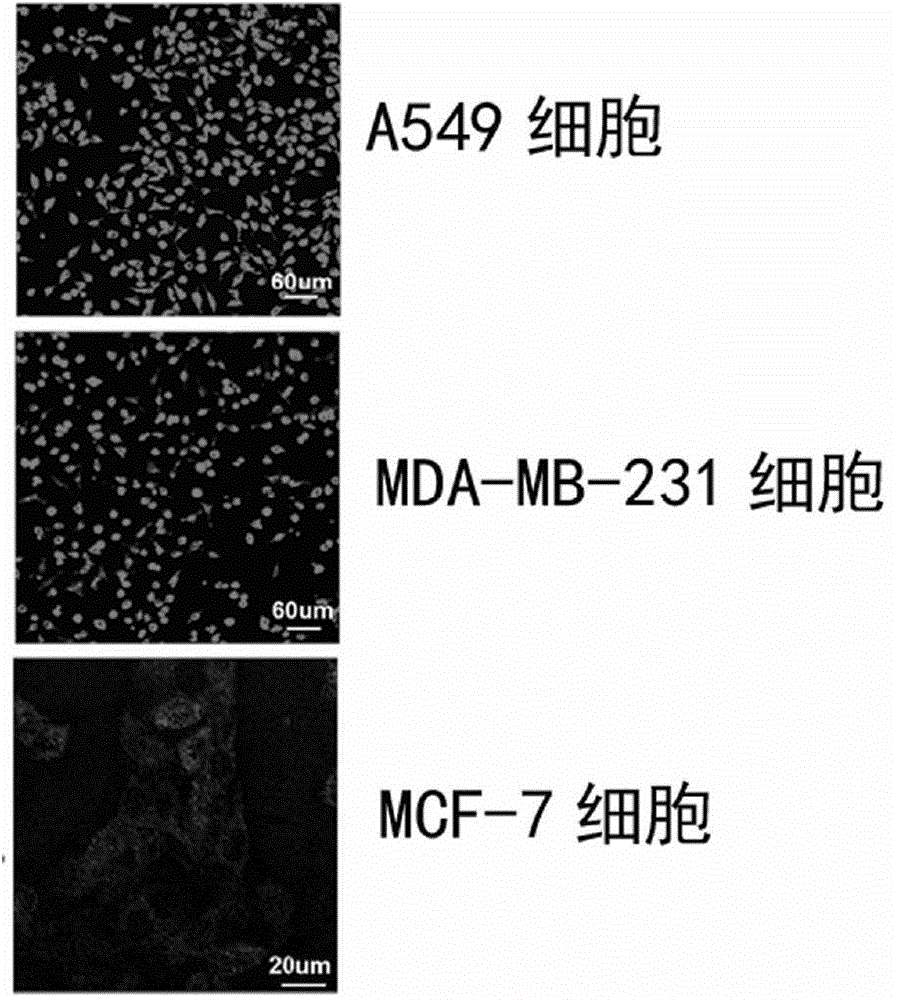
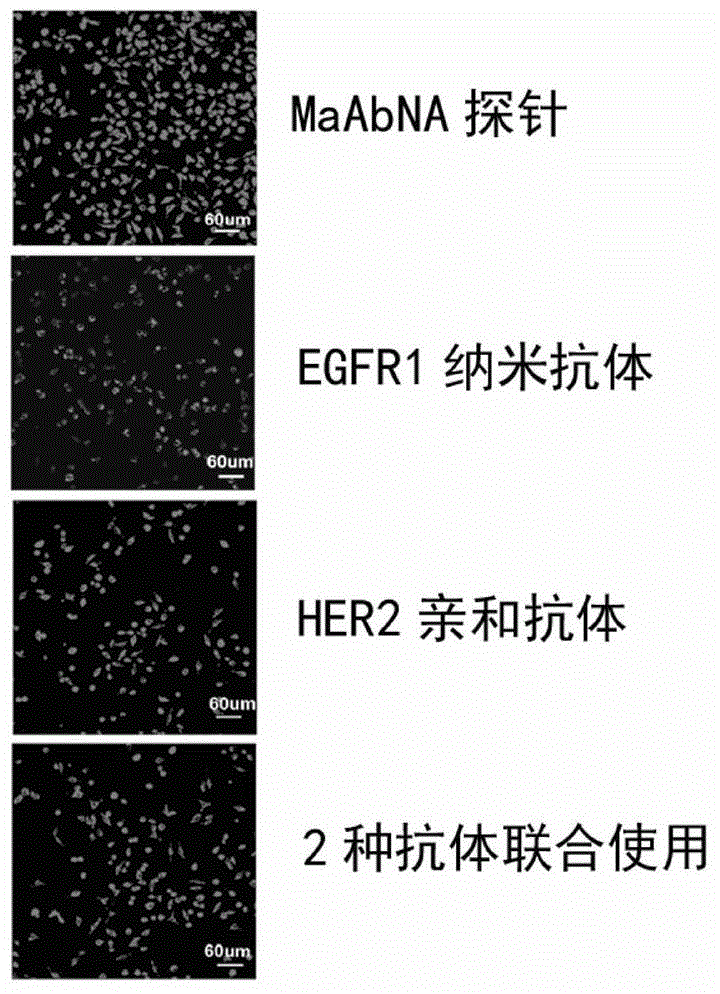
The present invention belongs to the field of bio-engineering pharmacy, protein polypeptide drugs and biomedical engineering, and relates to design and construction of a miniaturized fusion antibody providing high affinity for human epidermal growth factor receptors EGFR1 and HER2, and applications of the miniaturized fusion antibody in tumor targeting diagnosis and therapy, wherein the bispecific antibody is obtained by connecting a HER2 specific domain and a EGFR1 specific domain in series through a (G4S)3 connecting peptide and carrying out gene recombinant expression, the full-length of the fusion protein coding gene is 804 bp, 268 amino acids are coded, the molecular weight of the fusion protein is 29 kD, and the amino acid sequence is the sequence represented by SEQ ID No:1. Compared with combination of the EGFR1 and HER2 antibodies, the bispecific miniaturized antibody of the present invention provides significant in vitro and in vivo activity advantages, has advantages of low toxicity, being rapidly cleared in non-tumor sites in vivo, and the like, has high efficiency in targeting diagnosis and therapy, and can be used for tumor diagnosis and therapy.
Owner:CHINA PHARM UNIV
Uses for and article of manufacture including her2 dimerization inhibitor pertuzumab
InactiveUS20160175438A1Prolong progression-free survivalReduce riskOrganic active ingredientsAntibody ingredientsHER2 Positive Breast CancerProgression-free survival
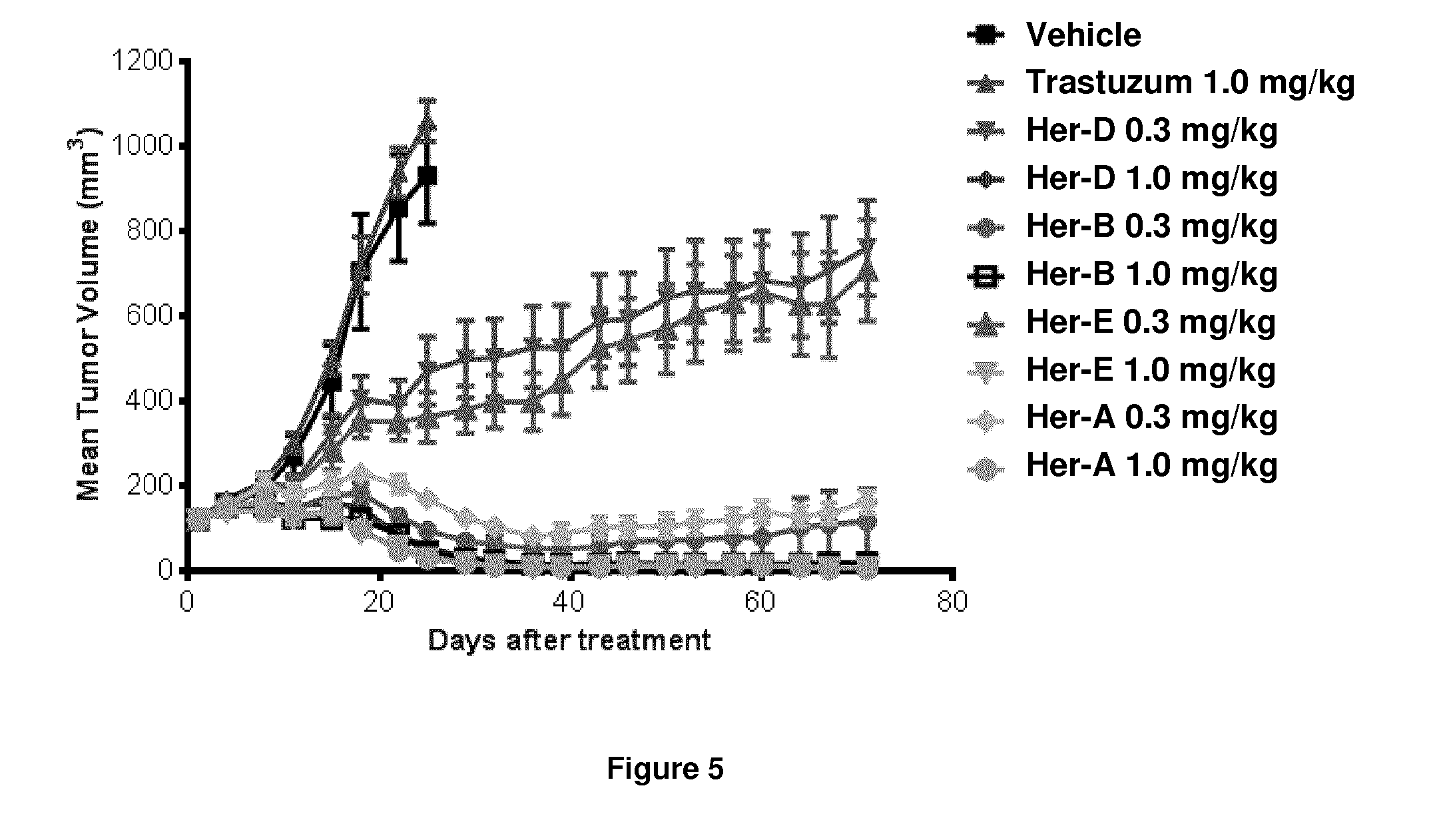
The present application describes uses for Pertuzumab, a first-in-class HER2 dimerization inhibitor. In particular, the application describes methods for extending progression free survival in a HER2-positive breast cancer patient population; combining two HER2 antibodies to treat HER2-positive cancer without increasing cardiac toxicity; and treating HER2-positive cancer by co-administering a mixture of Pertuzumab and Trastuzumab from the same intravenous bag.
Owner:GENENTECH INC
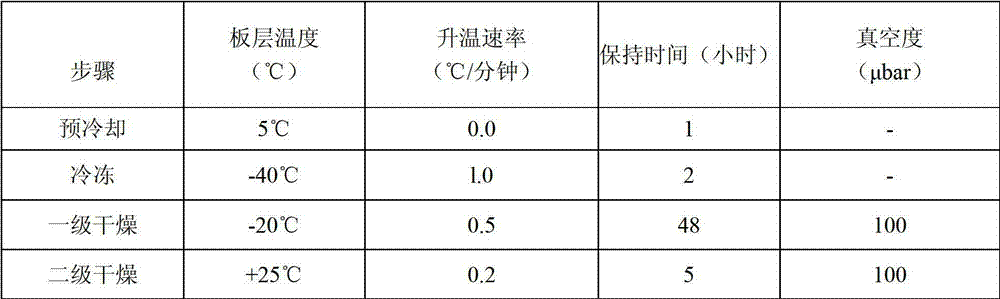
Lyophilized preparation of anti-human Her2 antibody
InactiveCN101721700AImprove stabilityEasy to storePowder deliveryAntibody ingredientsMedicineExcipient
The invention provides a preparation method of a lyophilized preparation of an anti-human Her2 antibody. The lyophilized preparation of the anti-Her2 antibody is prepared from the anti-Her2 antibody, a protective agent, excipient and buffer salts through lyophilization. The lyophilized preparation of the anti-Her2 antibody has the advantages of good stability, prolonged valid period, and easy storage and transportation.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG
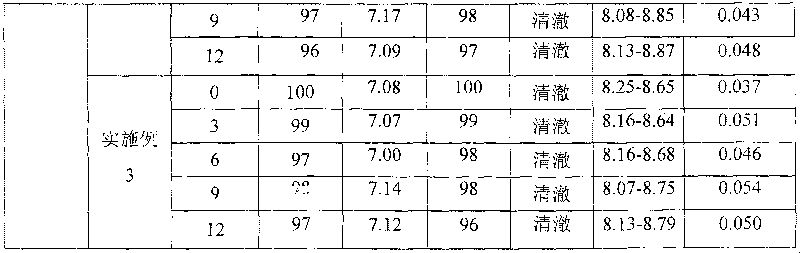
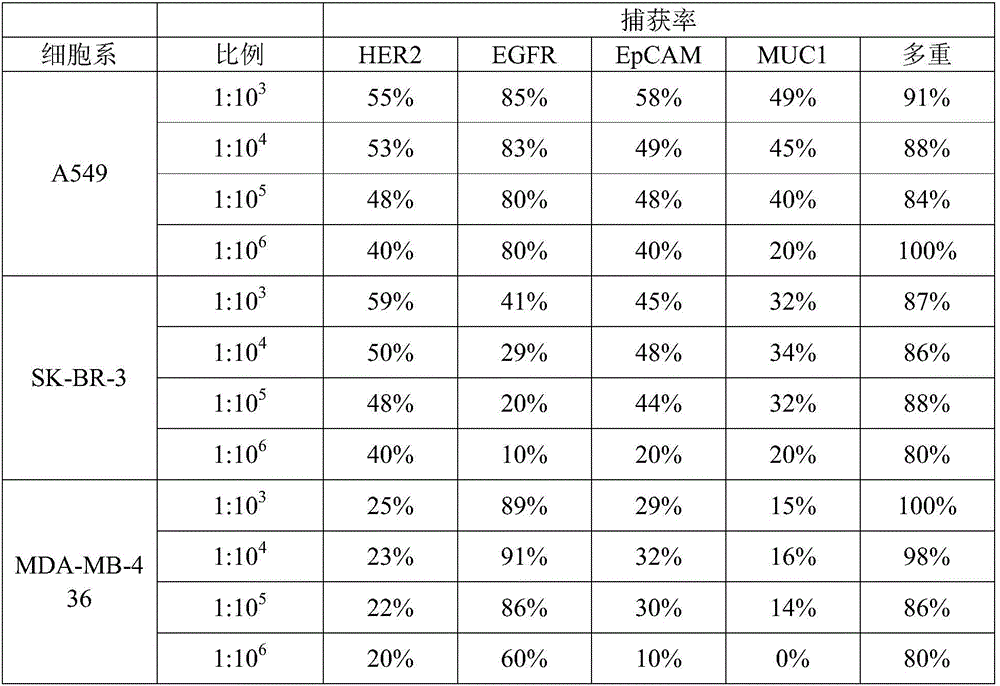
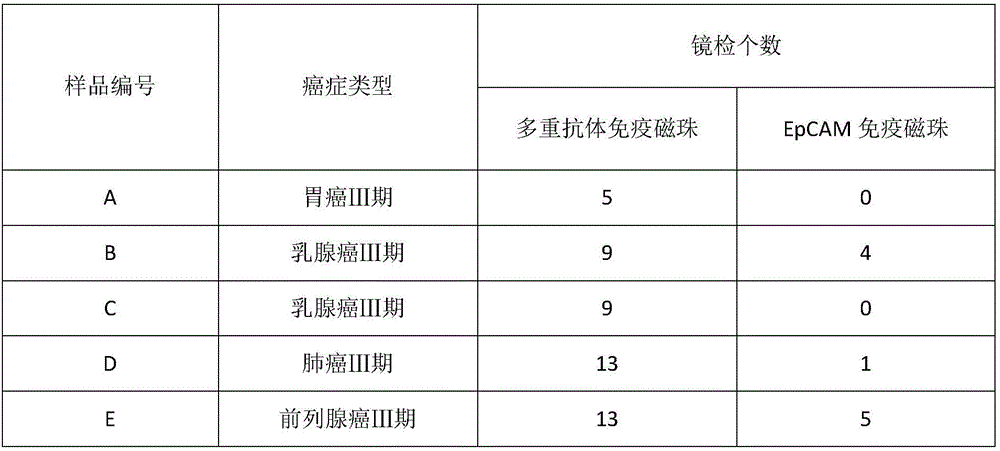
HER2, EGFR, EpCAM and MUC1 multiple antibody immunomagnetic bead and preparation method thereof
ActiveCN106366197AAvoid damageStrong specificityInorganic material magnetismCarrier-bound/immobilised peptidesMedicineMicrosphere
The invention provides a HER2, EGFR, EpCAM and MUC1 multiple antibody immunomagnetic bead and a preparation method thereof. The multiple antibody immunomagnetic bead includes a HER2 immunomagnetic bead, an EGFR immunomagnetic bead and an EpCAM immunomagnetic bead, and a HER2 antibody, an EGFR antibody and an EpCAM antibody are respectively coupled with magnetic microspheres to obtain the HER2 immunomagnetic bead, the EGFR immunomagnetic bead and the EpCAM immunomagnetic bead. The multiple antibody immunomagnetic bead is good in specificity and sensitivity, rapid in magnetic response, short in concentration time and high in capturing efficiency when being used for capturing CTCs (circulating tumor cells). The immunomagnetic bead is stable in nature, small in particle size, good in magnetic response and dispersibility, simple in preparation method and high in practicability.
Owner:SHANGHAI MAJORBIO BIO PHARM TECH
Gene expression markers of tumor resistance to HER2 inhibitor treatment
ActiveUS9551033B2Inhibition of activationNucleotide librariesMicrobiological testing/measurementAbnormal tissue growthResistant genes
Owner:GENENTECH INC
Gene expression markers of tumor resistance to her2 inhibitor treatment
ActiveUS20170073777A1Inhibition of activationMicrobiological testing/measurementDisease diagnosisWilms' tumorFhit gene
The present invention concerns markers of resistance of HER2 expressing tumors to treatment with HER2 inhibitors, such as HER2 antibodies, including trastuzumab.
Owner:GENENTECH INC
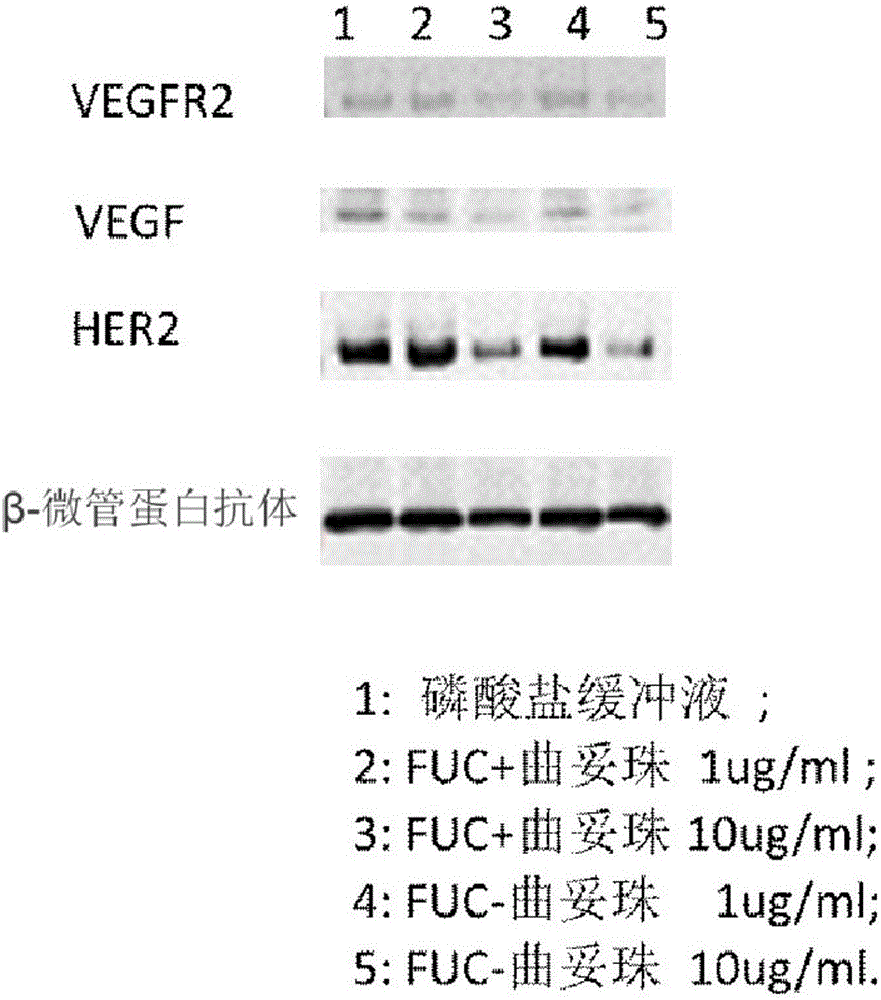
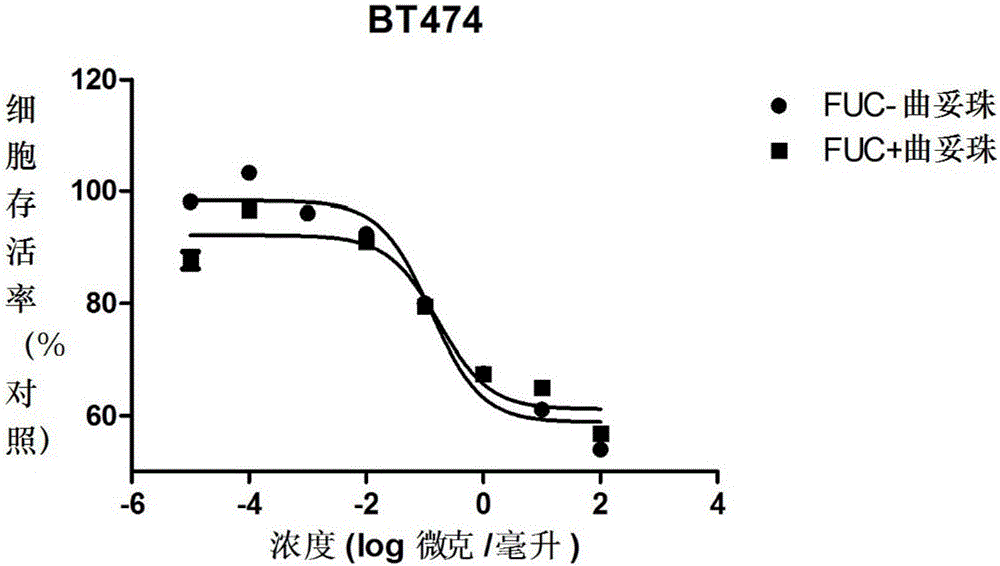
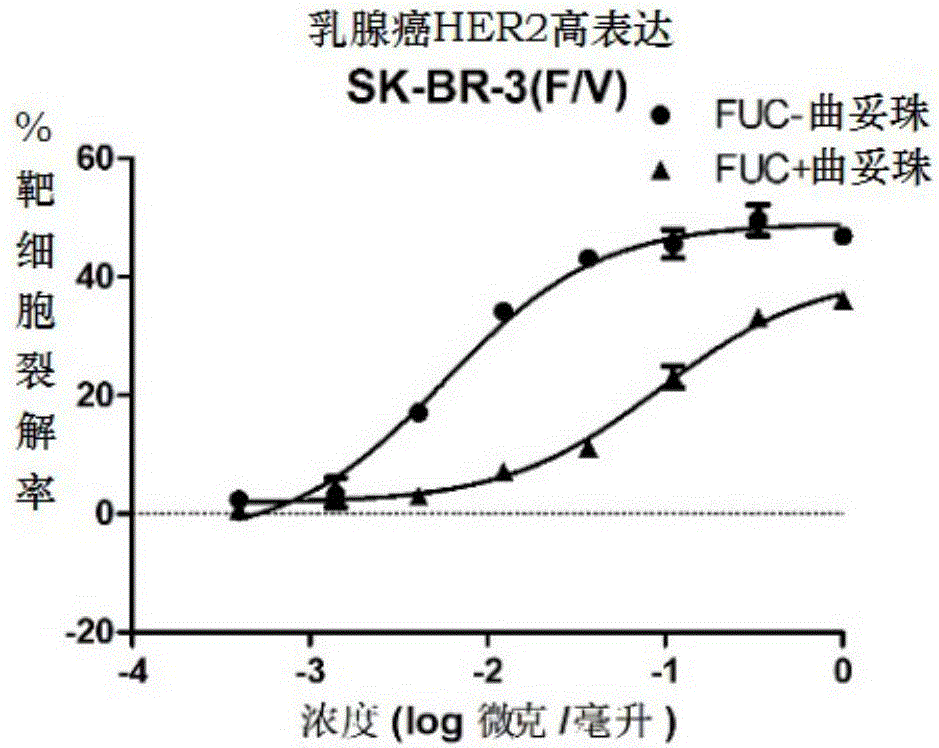
Fucose-removed anti-HER2 antibody and application thereof
InactiveCN106543286AGrowth inhibitionSuppress generationAntibody ingredientsImmunoglobulinsAnti her2BULK ACTIVE INGREDIENT
The invention provides a fucose-removed anti-HER2 antibody which is produced by cells with the FUT8 gene knocked out. The amino acid sequence of the fucose-removed anti-HER2 antibody is shown as SEQ ID NO: 1, and the nucleotide sequence is shown as SEQ ID NO: 2. Furthermore, the antibody comprises a CH2 structural domain; the amount of fucose in the CH2 structural domain is zero. The invention further provides a medicine with the fucose-removed anti-HER2 antibody serving as an active ingredient, and a reagent, a composition or a kit with the fucose-removed anti-HER2 antibody serving as an active ingredient. Compared with a fucose-containing anti-HER2 antibody, the antibody disclosed by the invention is higher in activity, and shows good tumor inhibition activity in both expression and low expression of herceptin-resistant HER2.
Owner:BIORAY LABORATORIES INC
Whole-human HER2 antibodies, and coding genes and application thereof
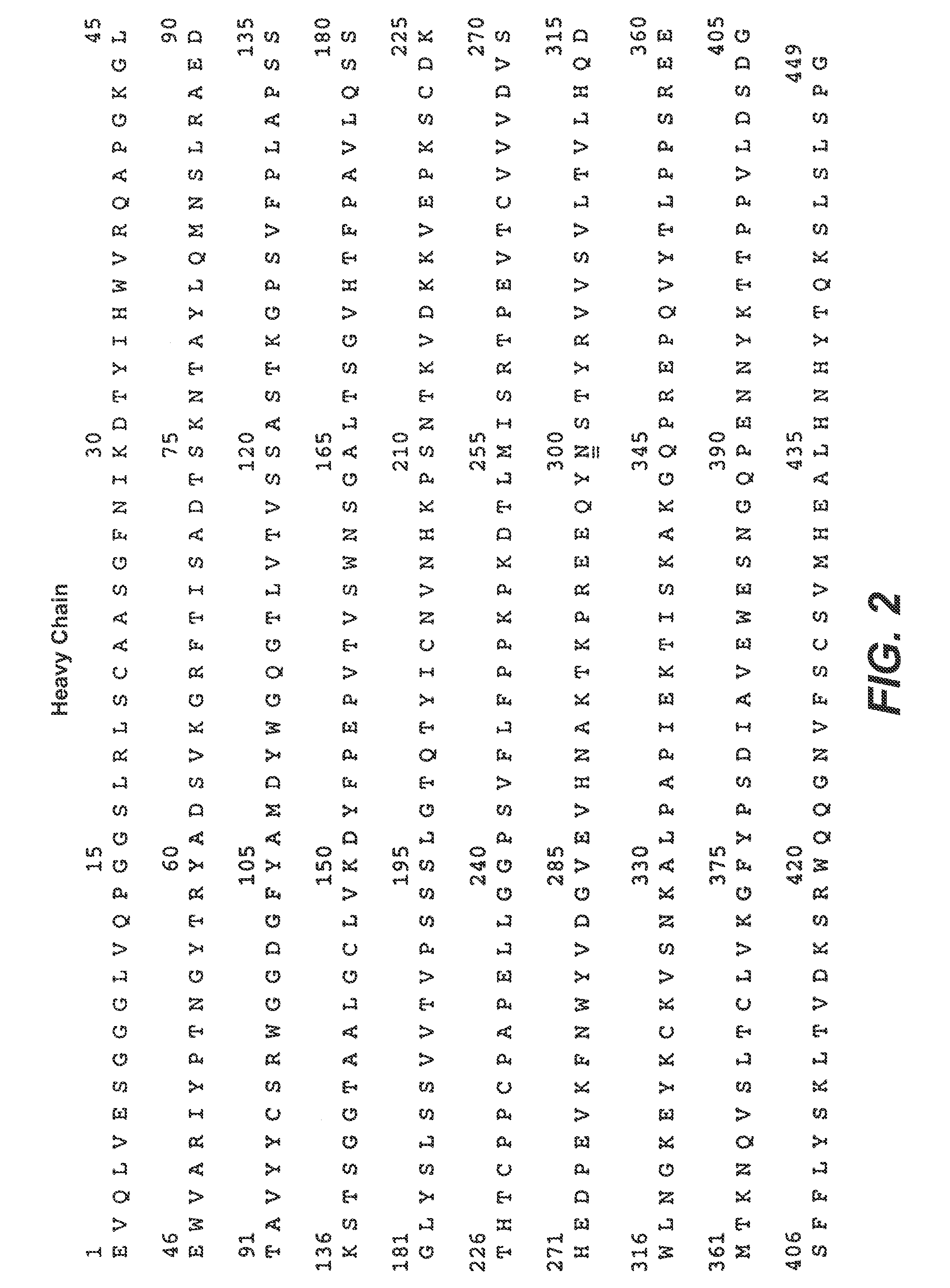
ActiveCN104530236AImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAbnormal tissue growthHeavy chain
The invention relates to the field of medicinal chemistry, and particularly relates to two whole-human HER2 antibodies, and coding genes and an application thereof. The invention provides the two whole-human HER2 antibodies, wherein an amino acid sequence of a heavy chain variable region of one HER2 antibody is SEQ ID NO:1, and an amino acid sequence of a light chain variable region is SEQ ID NO:2; an amino acid sequence of a heavy chain variable region of the other HER2 antibody is SEQ ID NO:10, and an amino acid sequence of a light chain variable region is SEQ ID NO:11. The whole-human HER2 antibodies can reduce transfusion reactions and immunogenicity, improve drug safety and have better pharmacokinetic characteristics. In addition, the whole-human antibodies can be combined with other HER2 positive tumor therapeutic agents for use in treatment of HER2 positive tumors.
Owner:GENOR BIOPHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com