HER2 antibody composition
A kind of composition, antibody technology, applied in the composition field of amino acid sequence variant
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
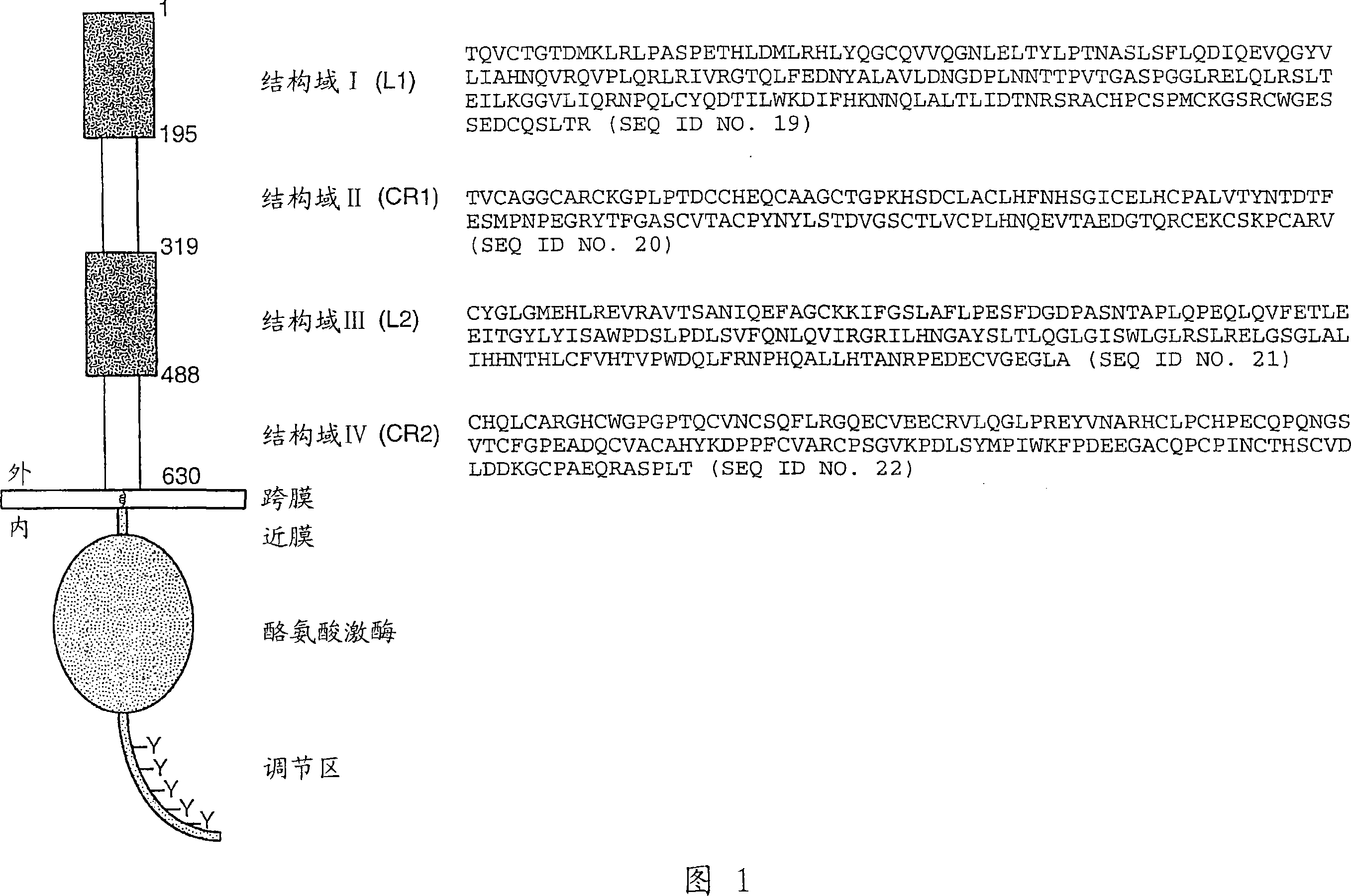
[0296] Identification of the composition of PERTUZUMAB
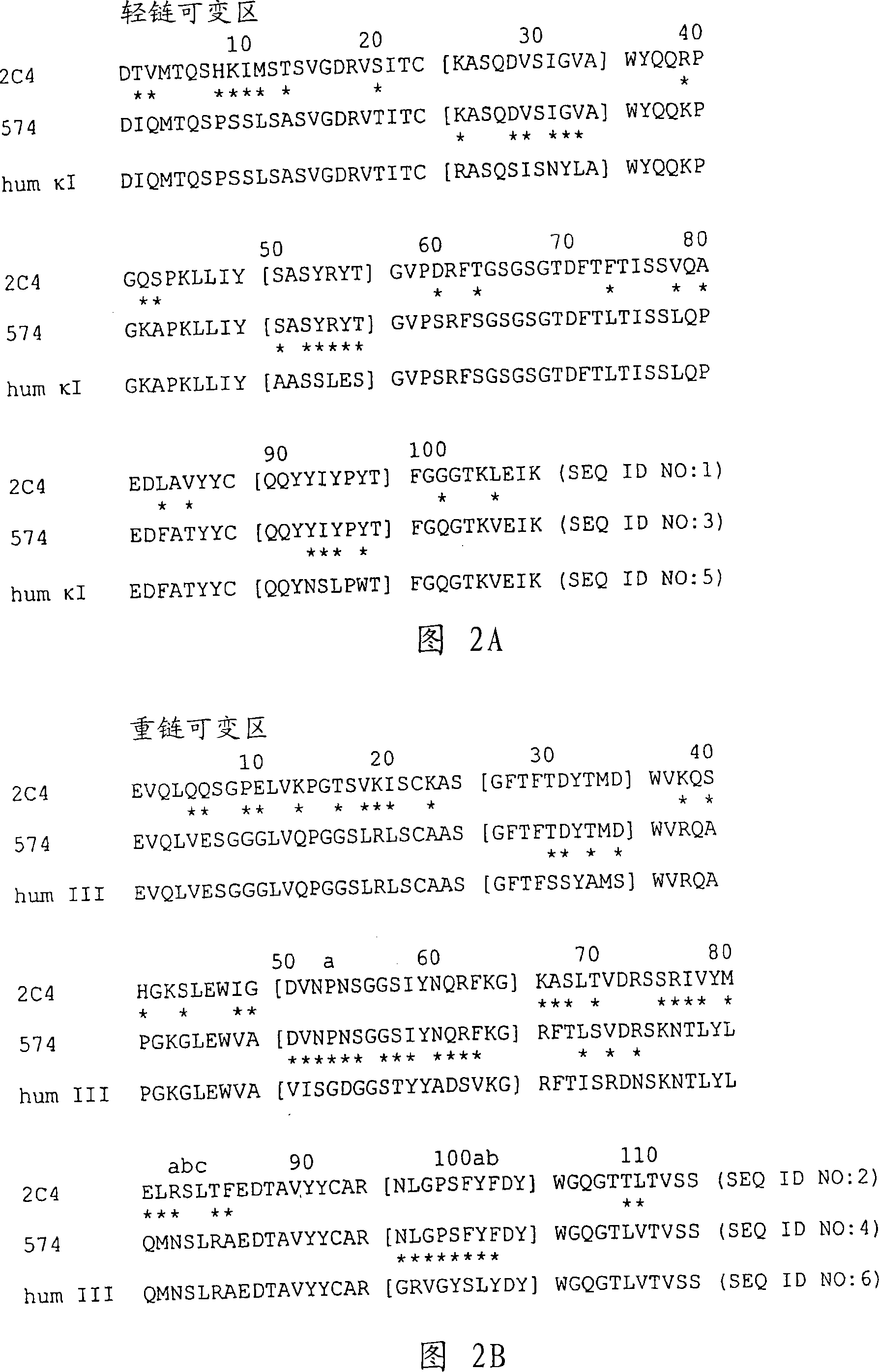
[0297] Pertuzumab is a recombinant humanized monoclonal antibody produced on the basis of human IgG1() framework. It contains two heavy chains (448 residues) and two light chains (214 residues). The two heavy chains are linked by two interchain disulfide bonds, and each light chain is attached to the heavy chain by one interchain disulfide bond. There is an N-linked glycosylation site at Asn-299 of both heavy chains in the Fc region of Pertuzumab. Pertuzumab with HERCEPTIN (Trastuzumab) differ in the epitope-binding regions of the light chain (12 amino acid difference) and heavy chain (30 amino acid difference). Because of these differences, Pertuzumab binds to a completely different epitope on the HER2 receptor. Binding of Pertuzumab to the HER2 receptor on human epithelial cells prevents it from forming complexes with other members of the HER receptor family (Agus et al., Cancer Cell 2:127-137 (2002)). By blocki...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com