Anti-tumor bispecific miniaturized antibody with double functions of targeting therapy and detection
An anti-tumor and anti-tumor drug technology, applied in the field of bioengineering, can solve the problems of high accumulation toxicity, low treatment efficiency, lack of target specificity, etc., and achieve high tumor treatment and detection efficiency, significant curative effect, and rapid clearance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Construction of recombinant expression plasmid pET22b-MaAbNA:
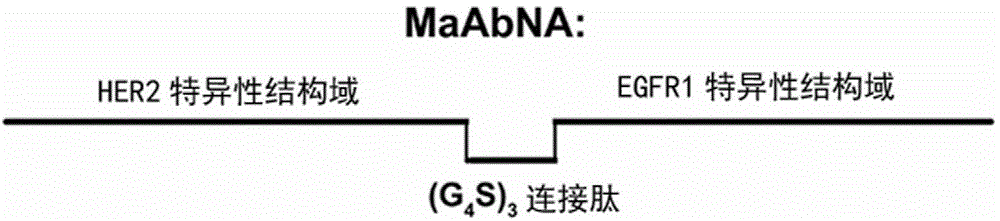
[0051] (1) The protein sequences of the specific domains of EGFR1 (gene coding sequence 1-372) and HER2 (gene coding sequence 420-804) were obtained from the literature, through (G 4 S) 3 The flexible peptide connects the HER2-specific domain and the EGFR1-specific domain in sequence, deduces the DNA sequence based on its overall amino acid sequence, and optimizes it according to the preferred codons of Escherichia coli, which is the MaAbNA gene coding sequence shown in SEQ ID No:2 . The recombinant gene sequence was synthesized by Nanjing GenScript Biotechnology Co., Ltd. after introducing restriction endonuclease (BamHI and NcoI) restriction sites at the 5' end and 3' end respectively. The gene synthesis adopts the chemical synthesis method, and its synthesis starts from the 3' end base of the DNA sequence shown in SEQ ID No: 2. The specific reaction steps are as follows:
[0052] ①Deprotection group ...
Embodiment 2
[0065] Expression, purification and renaturation of fusion protein:
[0066] (1) Transform the recombinant plasmid pET22b-MaAbNA into Escherichia coli BL21star TM (DE3), positive clones were screened with Amp-resistant SOB plates. After the transformed strain was cultured overnight at 37°C and 200 rpm, it was transferred to fresh LB medium at a ratio of 1:100, and continued to culture until the OD value of the bacterial solution was about 600, and the final concentration of 1 mmol / L IPTG was added to induce protein expression for 5 hours After the induction was terminated, the bacterial liquid was centrifuged and the bacterial cells were collected. After the induced recombinant bacteria were resuspended and ultrasonically lysed, the precipitate was dissolved in 8 mol / L urea solution.
[0067] (2) Use HisTrap Ni 2+ The column was used to purify the MaAbNA recombinant protein inclusion body. After loading the inclusion body solution, equilibration buffer, 5mmol / L, 10mmol / L, 2...
Embodiment 3
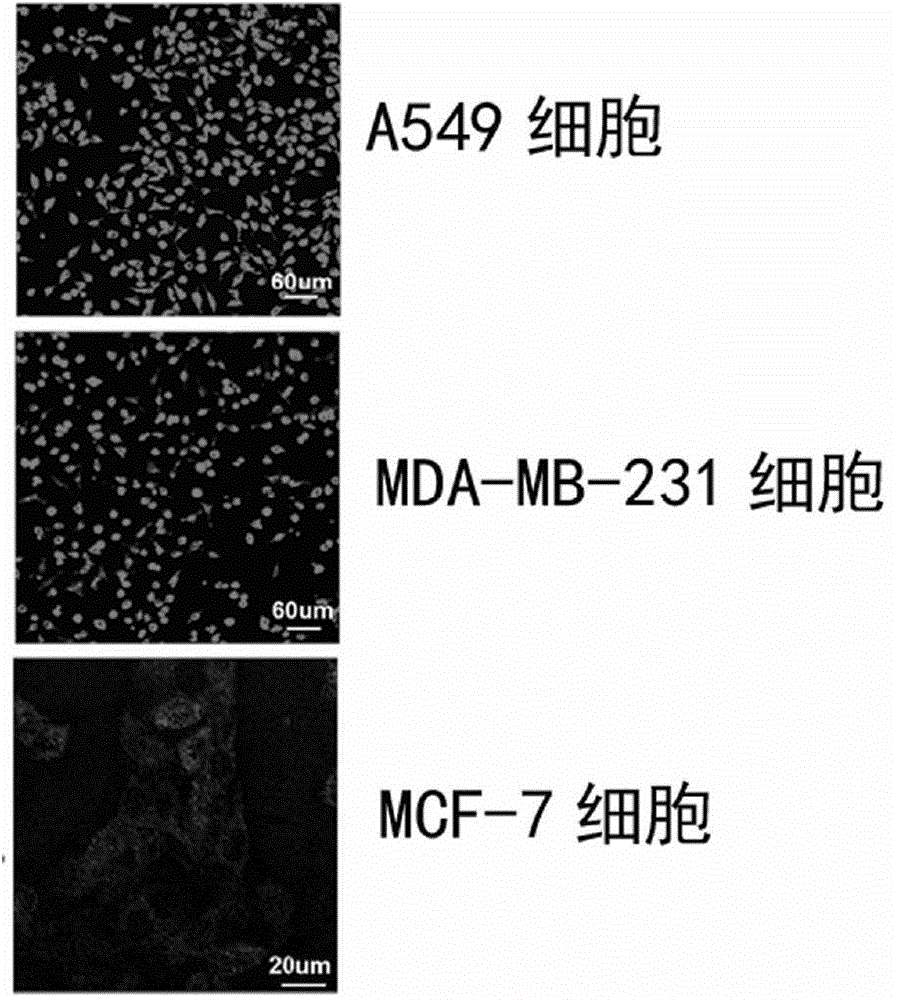
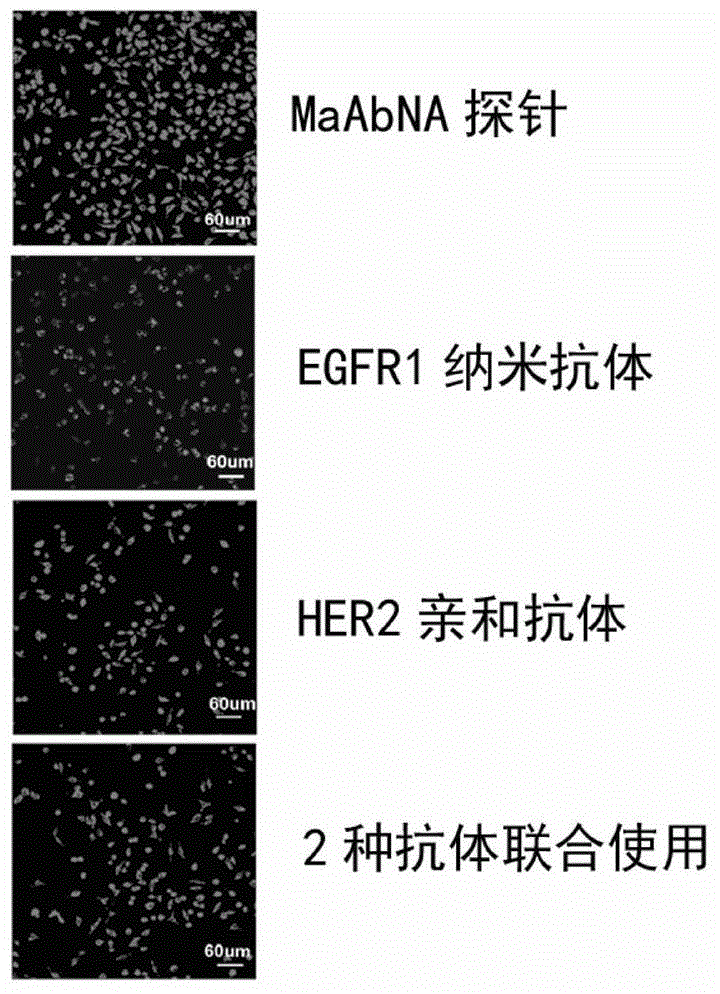
[0069] Synthesis of MaAbNA-rhodamine B in vitro diagnostic probe:
[0070] (1) Take 4.79mg of rhodamine B and 2.11mg of (3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and dissolve them in 1ml of dimethylformamide (DMF) Afterwards, the mixture was stirred and reacted for 2 hours at room temperature in the dark. After the reaction, 1.38 mg of N-hydroxysuccinimide (NHS) and 6 mg of MaAbNA were added to the solution to continue the reaction with stirring in the dark, and the reaction was stopped after 12 hours.
[0071] (2) The reaction product was applied to a Sephadex G-75 molecular sieve column, and washed with PBS buffer (pH 8.0) to collect red aggregated color bands. The collected product is the MaAbNA-rhodamine B in vitro diagnostic probe.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com