Fusion protein of Her2 antibody and interleukin 2 and application thereof
A technology of interleukin and fusion protein, applied in the field of fusion protein of anti-Her2 antibody and interleukin 2, which can solve the problems of HF clinical application limitation, low biological activity and low expression level, and achieve biological activity and yield Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
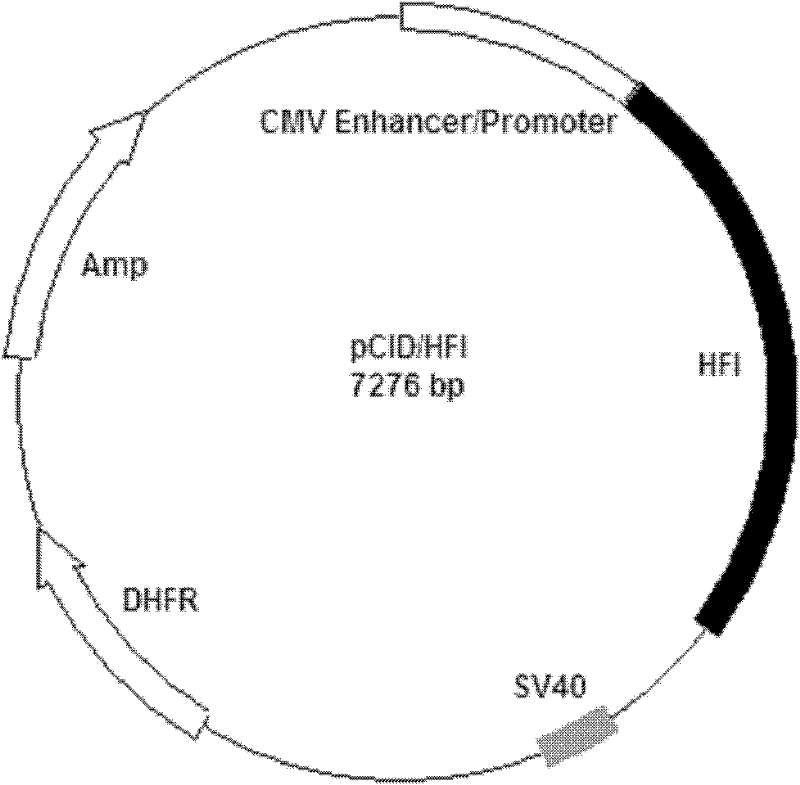
[0032] Example 1 Construction of Anti-Her2 Antibody-Interleukin 2 Fusion Protein Gene Expression Vector
[0033]Using the fusion protein gene vector pCID / HF containing anti-ErbB-2 scFv, Fc segment of human IgG1, and IL-2 as a template (the nucleotide sequence of the fusion protein in the vector is shown in SEQ ID No.5 in the sequence listing), Use primers P1 (its nucleotide sequence as shown in SEQ ID No.6 in the sequence listing) and P2 (its nucleotide sequence as shown in SEQ ID No.7 in the sequence listing) to PCR amplify the single Cloning of antibody heavy chain signal peptide gene sequence, ErbB2 antibody heavy chain variable region gene, Linker and light chain variable region gene and human antibody Fc gene fragment, the conditions are: denaturation at 95°C for 2 minutes; then denaturation at 94°C for 1 minute, annealing at 58°C 1min, 72°C extension for 2min, 30 cycles; finally 72°C extension for 10min. Insert the expression vector pCID with NheI and XhoI sites to obta...
Embodiment 2
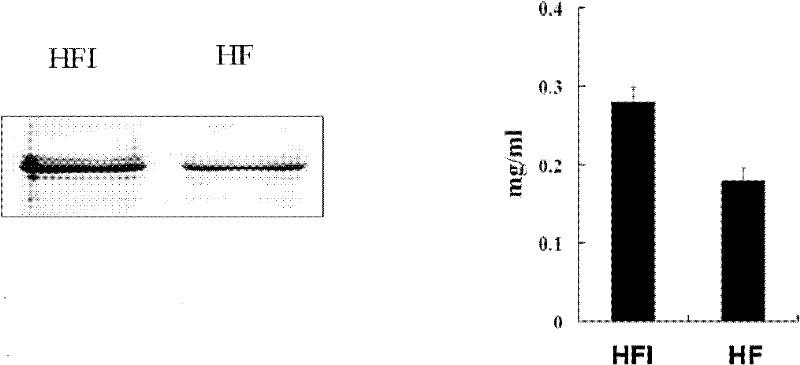
[0046] The screening of embodiment 2 engineering cell lines and the expression of HFI
[0047] CHO / dhfr- cells were cultured in IMDM medium containing 10% FCS, 0.1mol / L hypoxanthine, and 0.016mol / L thymidine. According to the instructions of Lipofectamine reagent, the recombinant vector PCI / HFI was transfected into CHO / dhfr- cells. After the transfected cells were cultured for 48 hours, the transient expression of the fusion protein was detected by the sandwich ELISA method, and the method was the same as before. The transfected cells were cloned and cultured by limiting dilution method, and the culture medium was containing 10% dialyzed FCS, 10 -6 MTX in DMEM medium. During the screening process, at different time points, the antibody expression level of each cell clone was detected by sandwich ELISA method. The specific process is as follows: Coat a 96-well plate with goat anti-human IgG (2 μg / ml), overnight at 4°C. After blocking with 2% bovine serum albumin for 1 h, th...
Embodiment 3
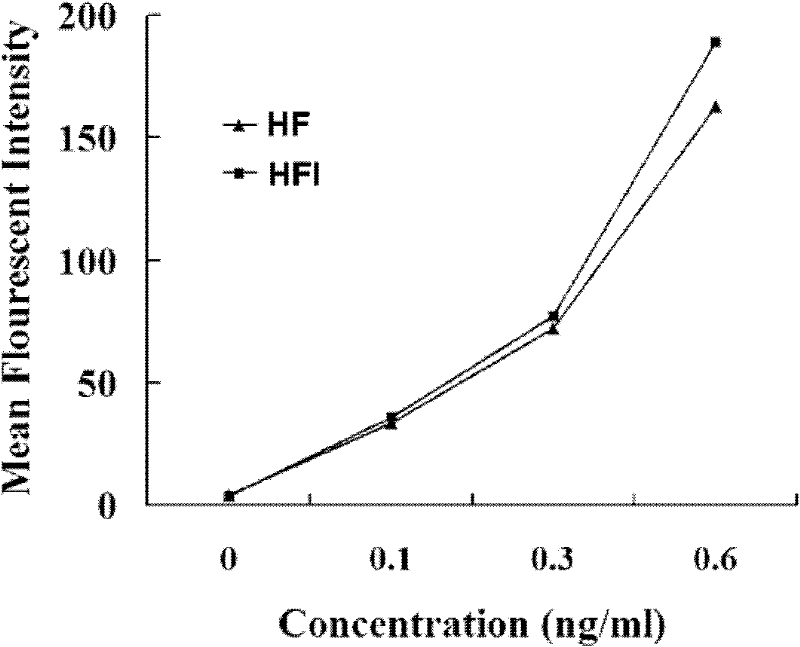
[0051] Example 3 HFI and HF antigen binding activity and biological activity detection experiment
[0052] Collect 1×10 6 SKBR3 cells were washed with PBS buffer, added purified HFI and HF, incubated for 30 minutes with ice bath shaking, washed 3 times with PBS, added FITC-labeled goat anti-human IgG, incubated for 30 minutes under ice bath shaking, washed with PBS Three times, analyzed on the machine (Becton Dickinson Company), and the cells not incubated with the culture supernatant of transfected cells were used as negative control.
[0053] The cultured CTLL-2 cells were washed 3 times with 1640 medium, after counting, 3 × 10 4 Inoculate each well in a 96-well plate, add purified HFI and HF at the same time, and use the doubly diluted IL-2 standard as a positive control. After culturing for 18 hours, 10 μl of MTT (5 mg / ml) was added, and the culture was continued for 4 hours. The cells were lysed with 10% SDS-0.01mol / L HCl, and the A490 value was measured.
[0054] The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com