Methods of treating her2-positive cancers using pd-1 axis binding antagonists and Anti-her2 antibodies
a technology of pd-1 axis binding antagonist and anti-her2 antibody, which is applied in the field of her2-positive cancer treatment, can solve the problems of refractory, exhaustion or tolerance to foreign antigens, etc., and achieve the effects of enhanced priming, activation, proliferation and/or cytolytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
HER2 T Cell Dependent Bispecific Antibody (HER2-TDB) for Treatment of HER2 Positive Cancers
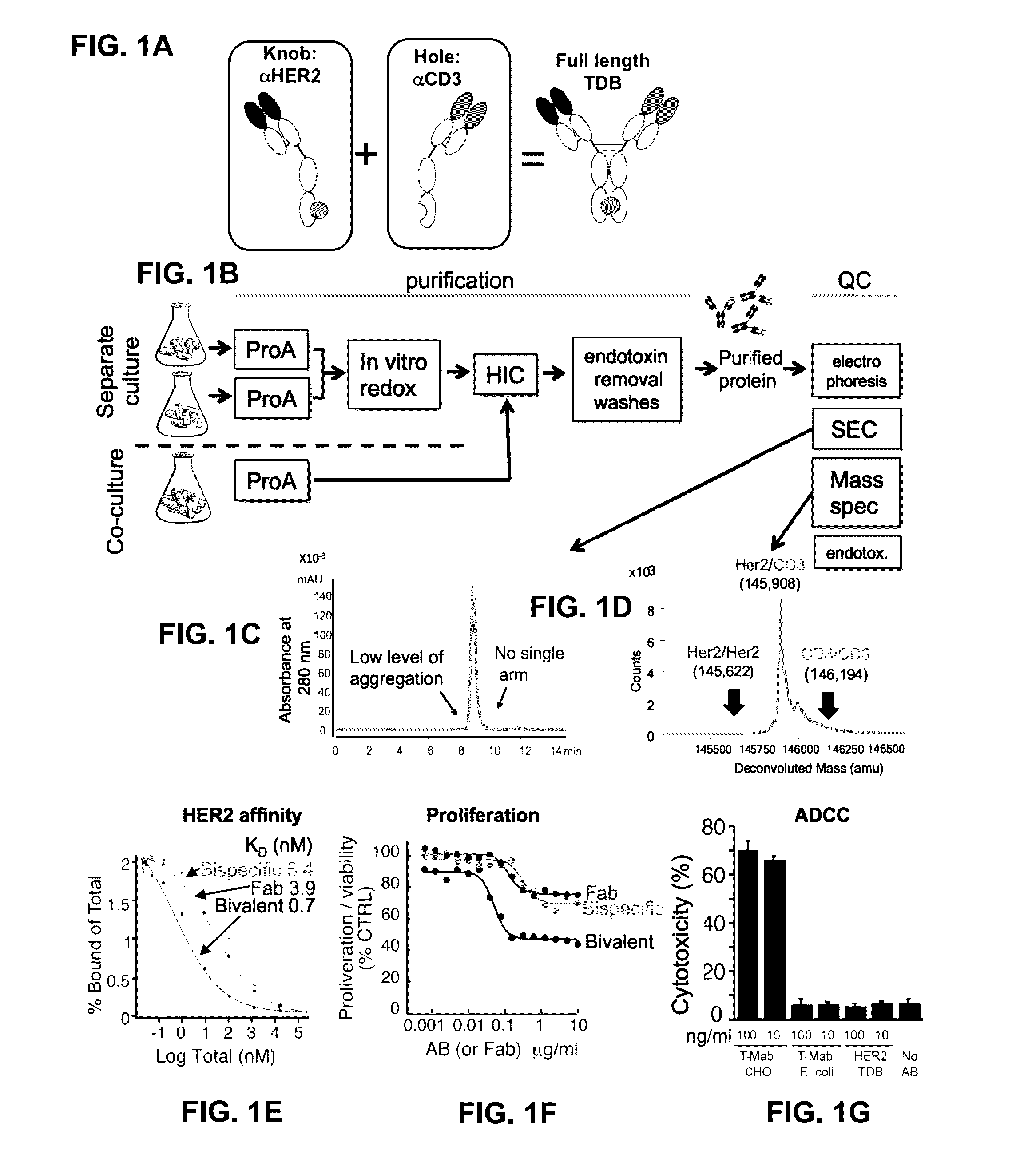
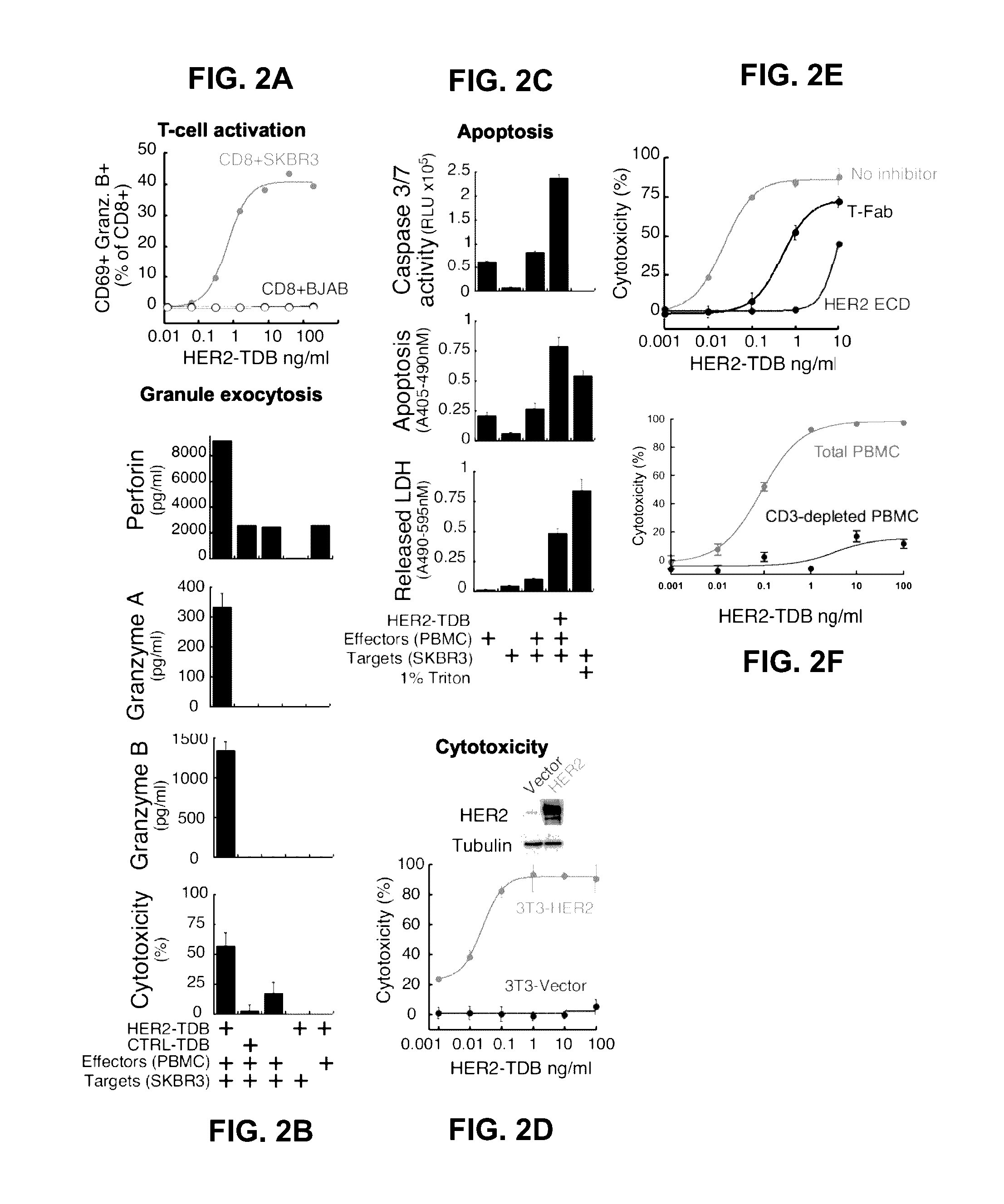
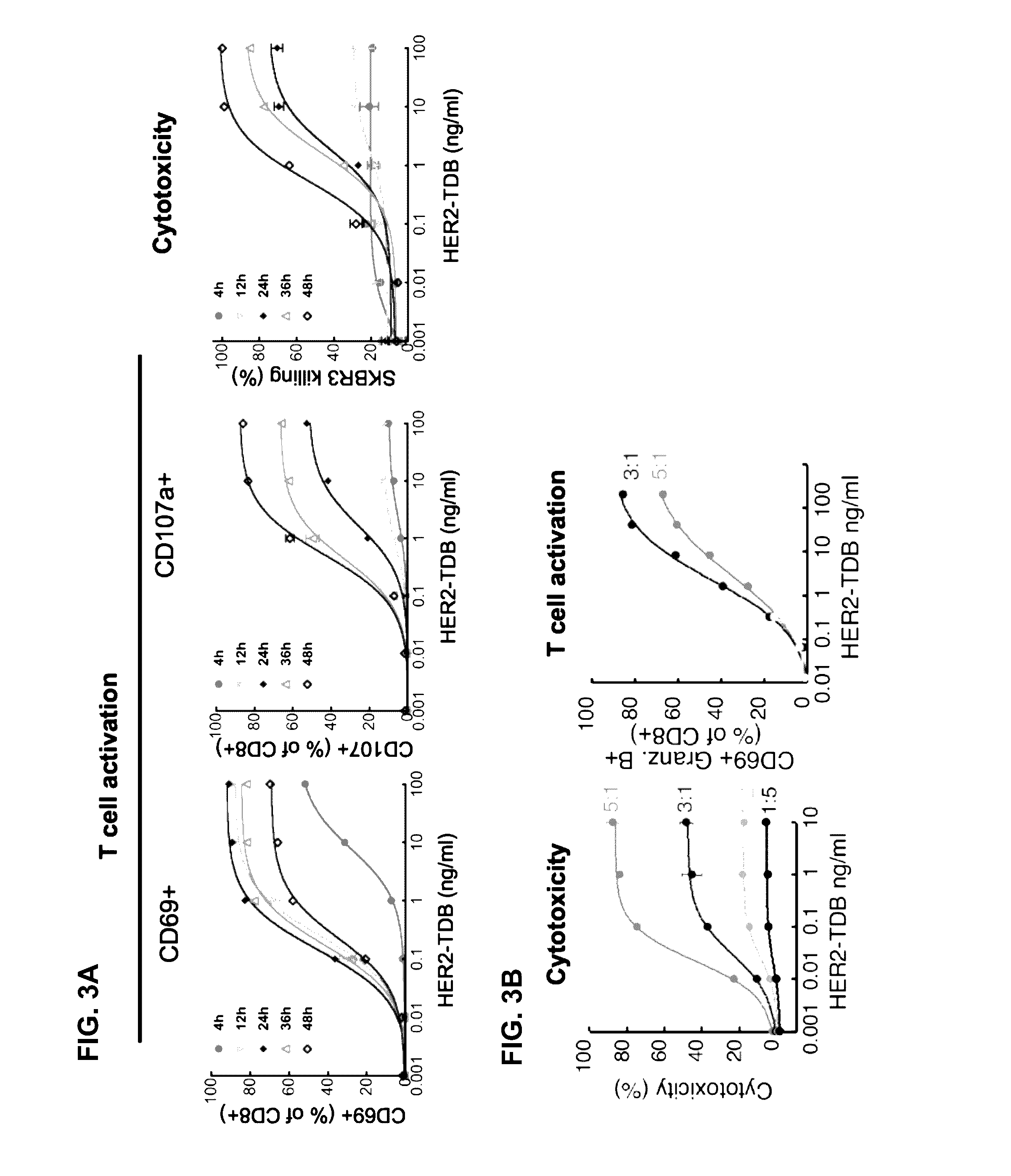
[0352]Based on recent clinical success of tumor immunotherapies that block immune suppressive mechanisms to restore T cell function, there is a profound interest in the clinical development of T cell targeted therapies. To meet this demand, described herein is a trastuzumab-based HER2 T cell dependent bispecific antibody (HER2-TDB). This full-length human IgG format bispecific antibody conditionally activated T cells resulting in lysis of HER2 expressing cancer cells at low picomolar concentrations Importantly, HER2-TDB was able to eliminate cells refractory to currently approved HER2 therapies. The potent anti-tumor activity of HER2-TDB was demonstrated using four model systems including MMTV-huHER2 and huCD3 transgenic mice. These results demonstrated inhibitory effect of PD-L1 expression on the activity of bispecific T cell recruiting antibodies. This resistance mechanism was reversed by an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tumor shrinkage | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com