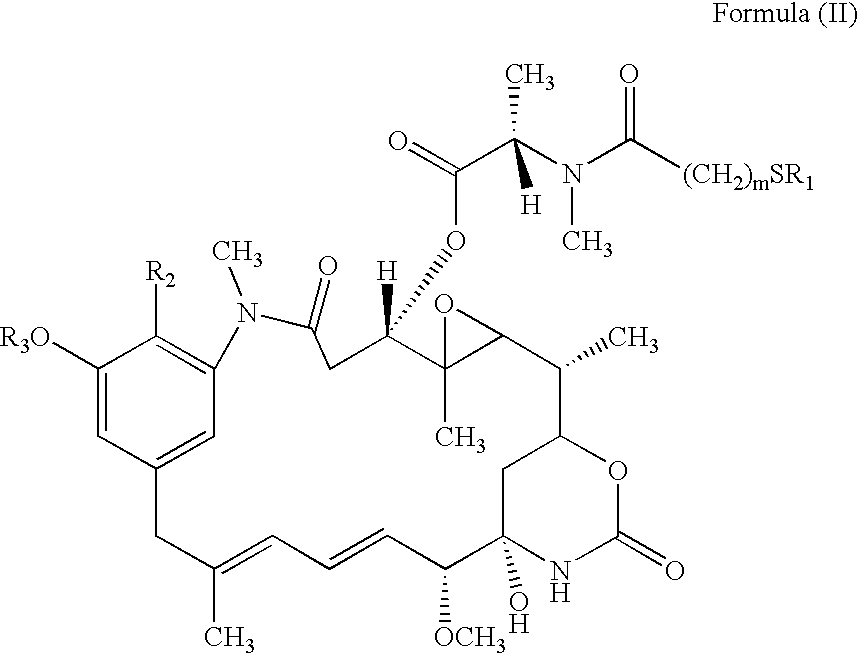
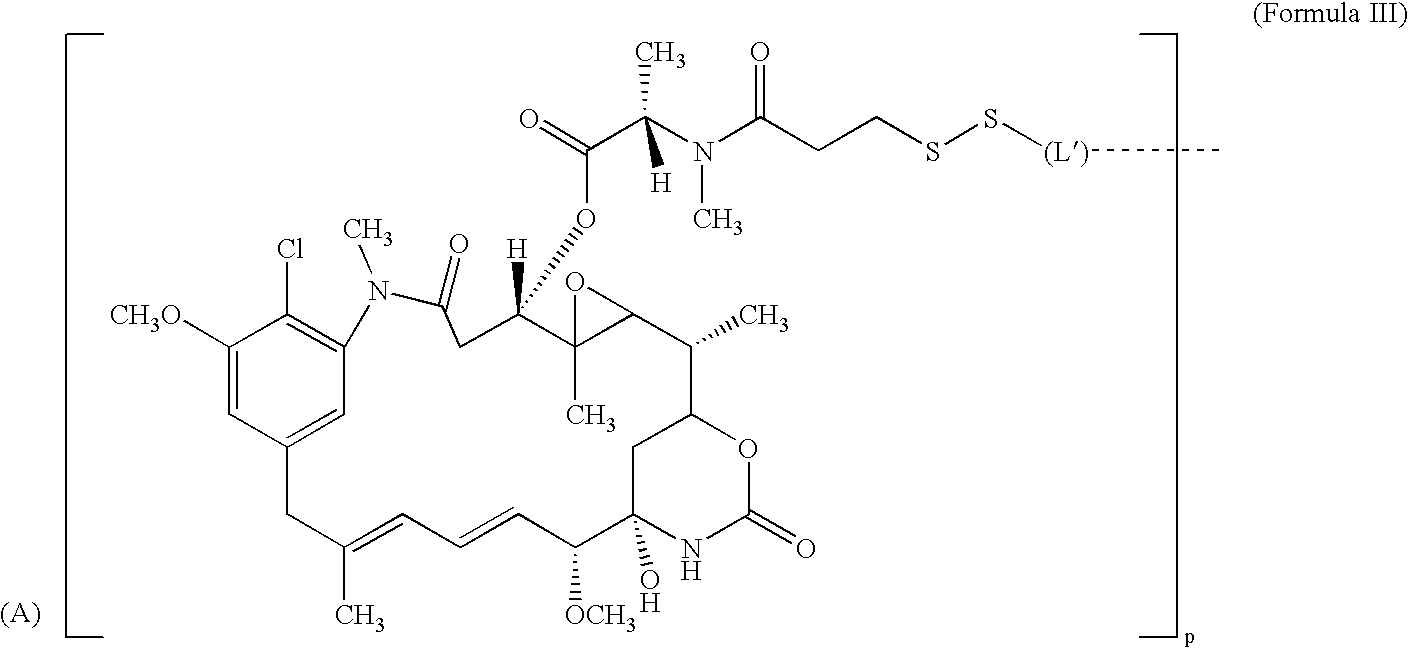
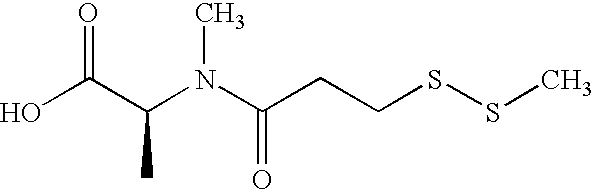
Cytotoxic CD44 antibody immunoconjugates
a technology of cytotoxic cd44 and antibody, which is applied in the field of new conjugates of antibodies with cytotoxic compounds, can solve the problems of difficult to achieve, inefficient internalization of antigens, and limited success of many of these approaches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
examples
[0076] 1. Material and Methods
[0077] 1.1. In vitro Cell Proliferation Assay
[0078] For determination of viable cells the Cell Titer 96.RTM. AQ.sub.ueous non-radioactive cell proliferation assay (Promega, Wisconsin, Wis.) was used. Five thousand cells per well were seeded into 96-well plates in 90 .mu.l medium without phenol red. Cells were allowed to settle for 1 to 3 h and then serial dilutions of the immunoconjugate in 10 .mu.l PBS were added. Cells without immunoconjugate served as negative control. Cells were incubated for 4 days at 37.degree. C. in a humified 5% CO.sub.2 atmosphere and then 20 .mu.l MTS / PMS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl- )-2H-tetrazolium / phenazine formazan) were added according to the manufacturer's recommendation. After an additional 1 to 4 h incubation at 37.degree. C., the absorbance at 490 nm was recorded using an ELISA plate reader. For each cell line, triplicates were analyzed. The percentage of the surviving cel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Toxicity | aaaaa | aaaaa |
| Bond | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com