Synthesis method of semaglutide
A technology of semaglutide and a synthesis method, which is applied in the field of peptide synthesis and preparation, can solve the problems of unfavorable environmental protection, high cost, difficult coupling and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1, a kind of synthetic method of semaglutide, comprises the following steps:
[0075] A. The fragments are condensed into various semaglutide intermediates, and the amino acid protecting groups are endowed with the following types:
[0076] (1) R-His(R1)-Ala-Glu(R2)-Gly-Thr(R4)-Phe-Thr(R4)-Ser(R4)-Asp(R5)-Val-Ser(R4)-Ser( Synthesis of R4)-Tyr(R4)-Leu-Glu(R2)-Gly-R3;
[0077] (2) Fmoc-Lys[[N6-[N-(17-carboxy(R3)-1-oxoheptadecyl-L-γ-glutamyl[2-(2-aminoethoxy)ethoxy]acetyl[2-(2-aminoethoxy)ethoxy Synthesis of ]acetyl] ]-OH;
[0078] (3) R-Gln-Ala-Ala-N6-[N-(17-carboxy(R3)-1-oxoheptadecyl-L-γ-glutamyl[2-(2-aminoethoxy)ethoxy]acetyl[2-(2- Synthesis of aminoethoxy)ethoxy]acetyl]-Lys-Glu(R2)-Phe-R3;
[0079] (4) Synthesis of R-Ile-Ala-Trp(R6)-Leu-Val-Arg(R7)-Gly-Arg(R7)-Gly-R3;
[0080](5) R-Gln-Ala-Ala-N6-[N-(17-carboxy(R3)-1-oxoheptadecyl-L-γ-glutamyl[2-(2-aminoethoxy)ethoxy]acetyl[2-(2- Synthesis of aminoethoxy)ethoxy]acetyl]-Lys-Glu(R2)-Phe-Ile-Ala-Trp(R6)-...
Embodiment 2
[0094] Example 2, in the synthesis method of semaglutide described in Example 1: the coupling system used in the synthesis of the 6 polypeptide fragments in step A is selected from:
[0095] Single condensing agent: DIC, DCC, EDC, chloroacetyl chloride, azide, TBTU, PyBOP, HBTU, HATU;
[0096] Two-system condensing agent: DIC / HOBT, DCC / HOBT, EDC / HOBT.
Embodiment 3
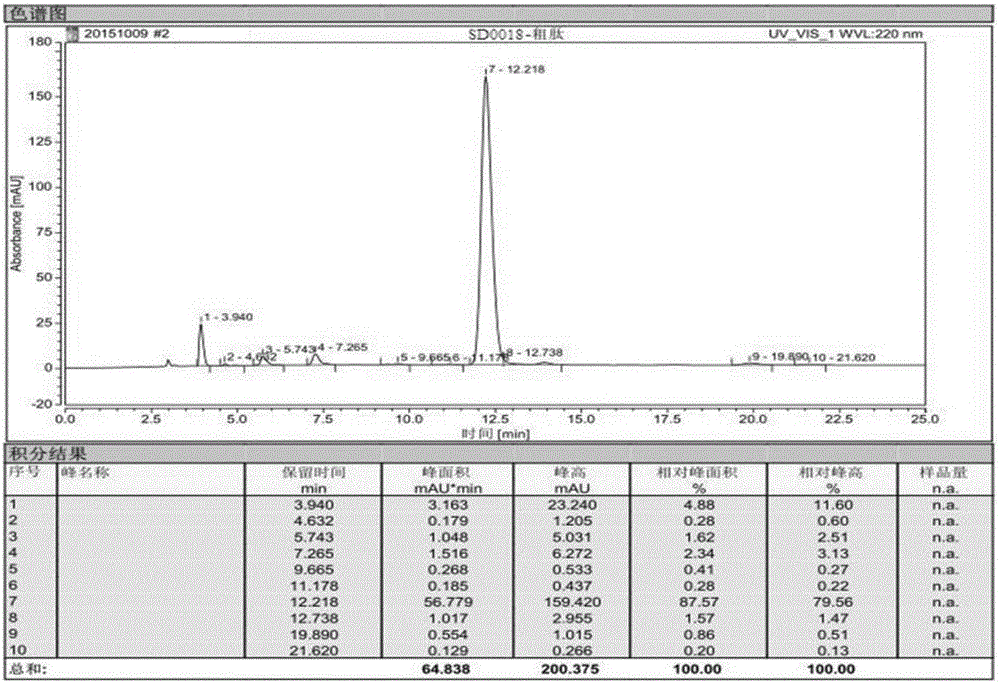
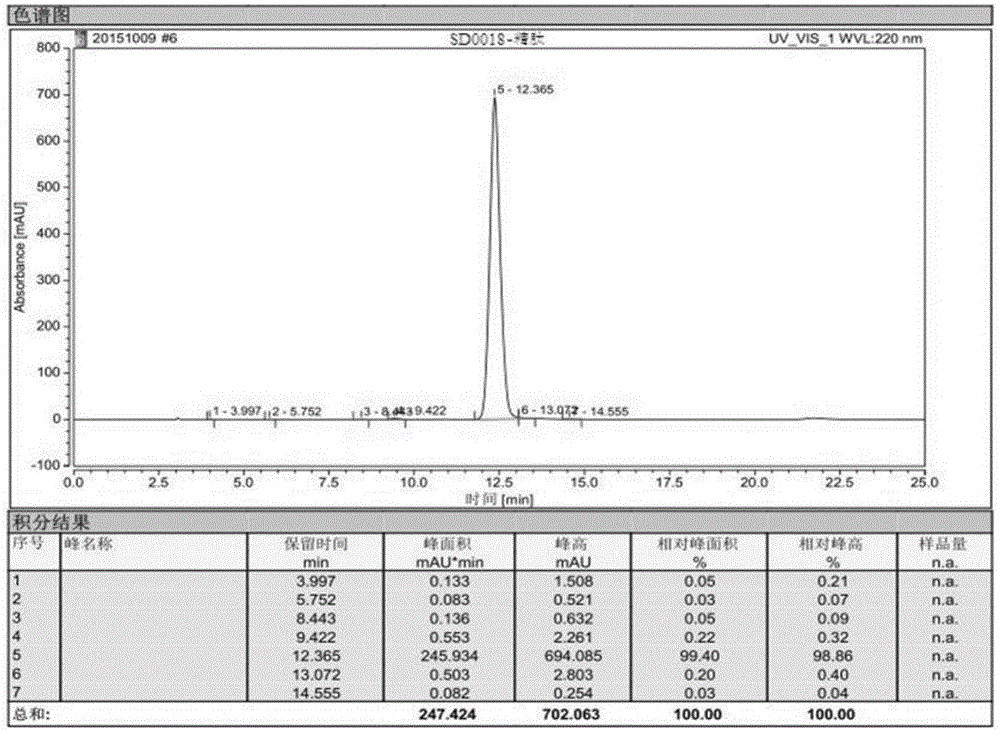
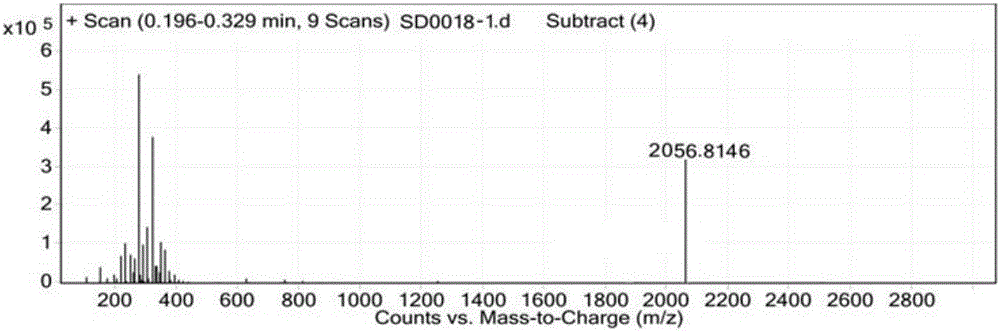
[0097] Example 3, in the synthesis method of semaglutide described in Example 1 or 2: the crude semaglutide is processed by purification and freeze-drying methods to obtain the semaglutide product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com