Glycosyl transferase mutant and method for catalytically synthesizing rebaudioside M by using glycosyl transferase mutant
A technology of glycosyltransferase and mutant, applied in the field of bioengineering, can solve the problems of poor water solubility and low efficiency, and achieve the effects of increasing yield, high catalytic efficiency and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 Glycosyltransferase UGT76G1 mutation site screening
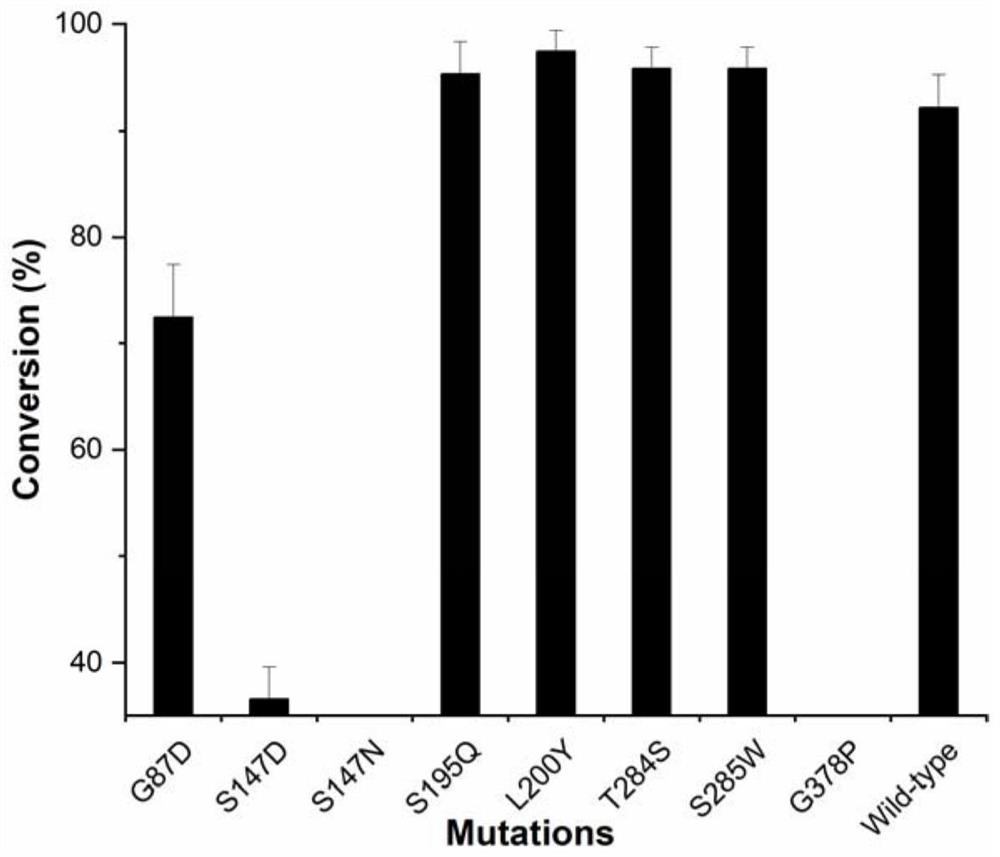
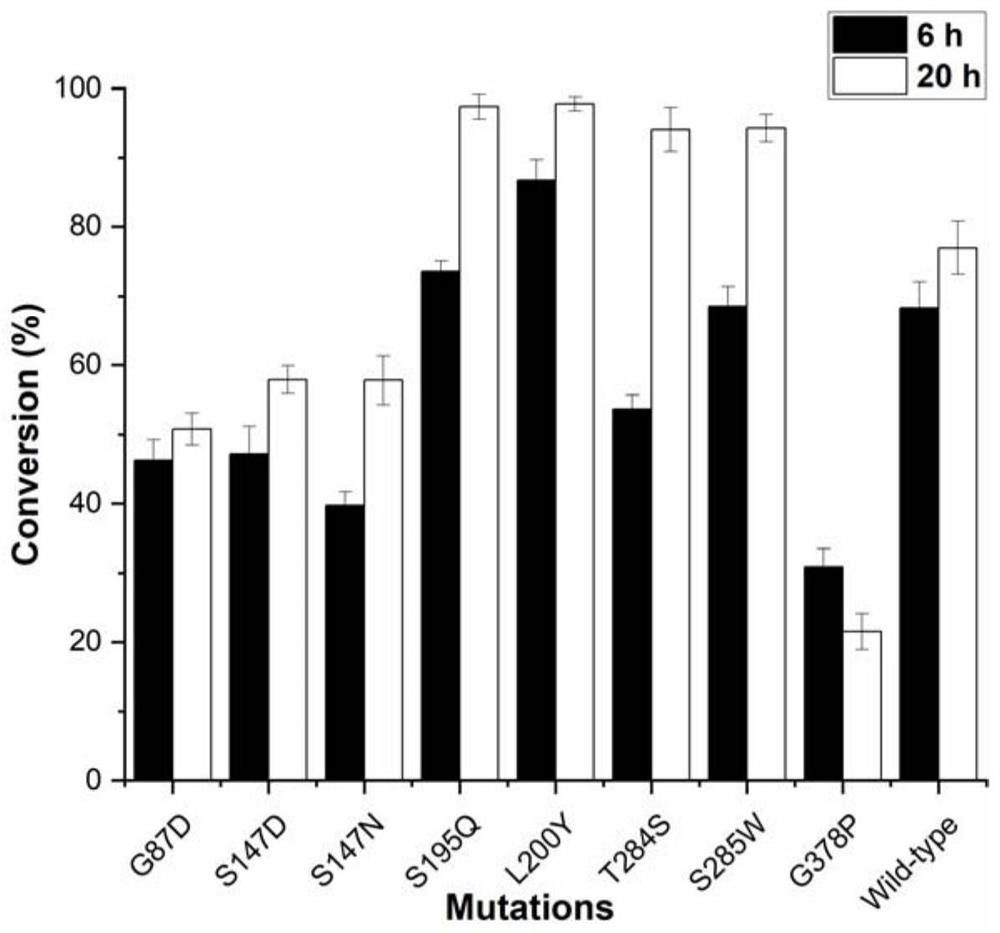
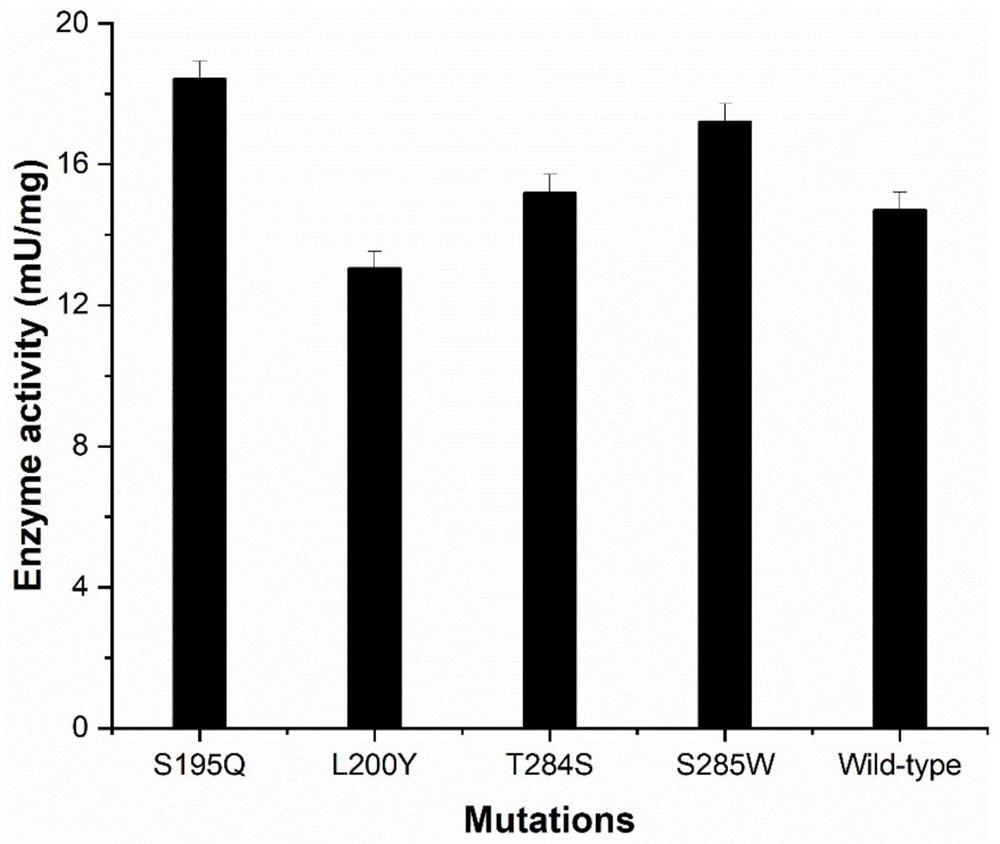
[0060] Transformation by directed evolution theory method. A crystal structure was selected and docked to obtain a complex model. A single-point mutation was performed on the amino acid residues in the structure and around RebE. The distance (Distance) between the NE2 of the key residue of the active center His25 in the structure and the O atom of the C13 glycoside of the substrate RebE was determined. Sort according to the value of affinity (Affinity). Combined with Affinity and Distance, the screened mutants are G87D, S147D, S147N, S195Q, L200Y, T284S, S285W, and G378P, as shown in Table 1.
[0061] Table 1 Mutant affinity value data
[0062]
[0063] Note: Affinity stands for affinity; Distance stands for distance between atoms; vdw stands for van der Waals force.
Embodiment 2
[0064] The construction of embodiment 2 double enzyme expression system
[0065] Select the double-gene expression vector pRSFDuet-1, insert the stevia-derived glycosyltransferase UGT76G1 at the NdeI / XhoI site of the vector, the nucleotide sequence is shown in SEQ ID NO: 2; insert sucrose at the NcoI / EcoRI site respectively Synthase StSUS1 or sucrose synthase McSuSy, the nucleotide sequences of which are shown in SEQ ID NO: 11 and SEQ ID NO: 12 constitute two corresponding recombinant expression plasmids, and the recombinant plasmids are respectively introduced into Escherichia coli BL21 (DE3) Transformation is carried out to obtain two double-enzyme co-expression recombinant strains.
Embodiment 3
[0066] Example 3 Preparation of Glycosyltransferase UGT76G1 Mutant
[0067] Using PCR amplification technology, the wild-type UGT76G1-StSUS1 recombinant plasmid was used as template DNA to carry out base-directed mutation.
[0068] Primers for G87D site-directed mutagenesis:
[0069] Forward primer: 5'-CCGCTGGCG GAC ATGCGTATTCCGATTATCAACGAA-3' (the underline is the mutant base), as shown in SEQ ID NO:13.
[0070] Reverse primer: 5'-AATACGCAT GTC CGCCAGCGGGCCGTGGGTCGGCAG-3' (the underline is the mutated base), as shown in SEQ ID NO:14.
[0071] Primers for S147D site-directed mutagenesis:
[0072] Forward primer: 5'-CTGATGACC GAC AGCCTGTTCAATTTTCATGCCCAC-3' (the underline is the mutant base), as shown in SEQ ID NO:15.
[0073] Reverse primer: 5'-GAACAGGCT GTC GGTCATCAGGACCAGGCGACGCAG-3' (the underline is the mutant base), as shown in SEQ ID NO:16.
[0074] Primers for S147N site-directed mutagenesis:
[0075] Forward primer: 5'-CTGATGACC AAC AGCCTGTTCAATTTTCATGCC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com