D-psicose 3-epimerase mutant with improved catalytic efficiency
A technology of epimerase and allulose, applied in the field of enzyme engineering, to achieve the effect of improving catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation method of Dorea sp.DPEase enzyme mutant
[0027] Construction of the recombinant plasmid pET22b-dore-dpe: According to Dorea sp.CAG:317 (accession number: CBGJ010000047.1), the D-psicose 3-epimerase gene fragment BN605_00564 was synthesized and linked to pET-22b (+) between the restriction sites Nde I and Xho I to obtain the recombinant plasmid pET22b-dore-dpe.
[0028] Construction of pET-22b(+)-A38E / G105A mutant plasmid: Using pET22b-dore-dpe plasmid as a template, the A38E / G105A site-directed mutation was introduced by PCR. The sequencing verification results showed that there was no randomness except the required mutation sites. Mutation, so the mutant plasmid pET-A38E / G105A was successfully constructed.
[0029] The mutation primers are as follows: (the underline is the mutation point)
[0030] A38E-F: 5’-GAAATCGGTGCT GAA CCATTACCGG-3’
[0031] G105A-F: 5’-CCATTTGATC GCC GGCGCTCTCTATG-3’
[0032] dore-R: 5’-GGTGTGTTTCCAATCCAACATATATTTC-3’
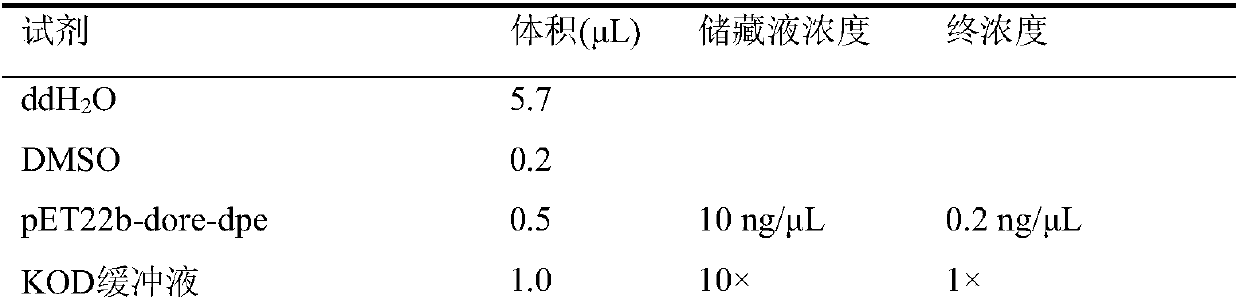
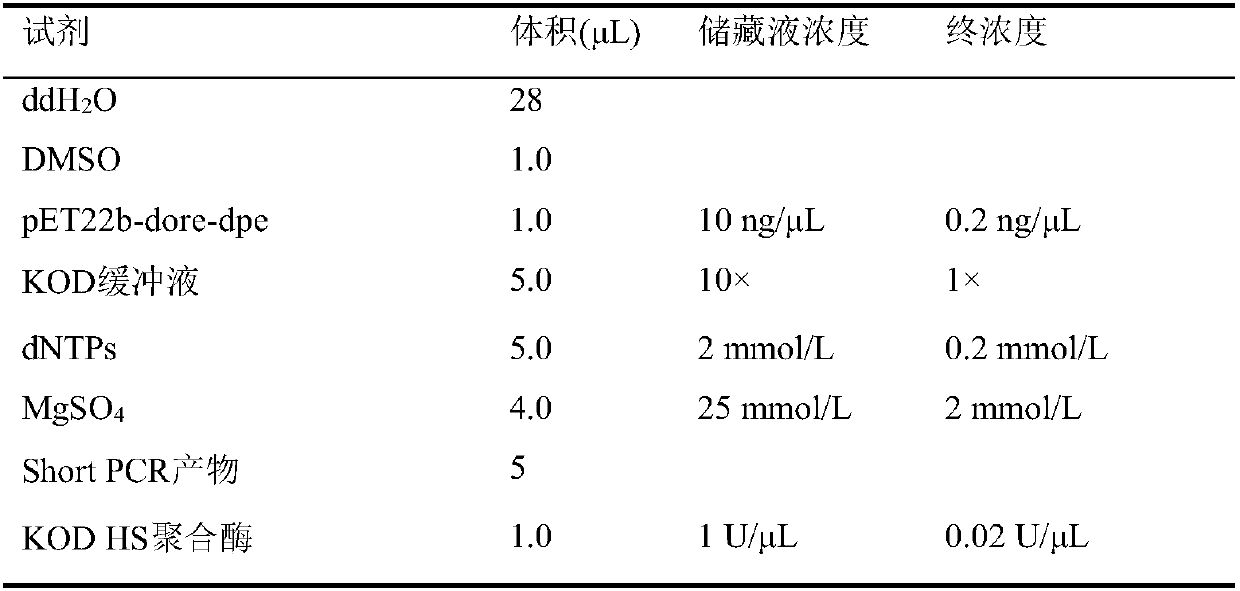
[0033] Sh...
Embodiment 2
[0041] Example 2: The expression and purification method of the mutant enzyme of Dorea sp. DPEase.
[0042] Transform the mutant plasmid pET-22b(+)-A38E / G105A verified by sequencing into E. coli BL21(DE3) cells, pick the positive transformants and shake them overnight in LB medium at 37°C and 200rpm, and then insert them into LB culture Incubate at 37°C for 3-4 hours to an OD value of 0.6-0.8, then cool to 30°C, add IPTG to a final concentration of 0.6mM and induce 6 hours.
[0043] The fermentation broth was centrifuged at 4°C and l0000 rpm for 20 min, and the bacteria were collected. Add 20mL buffer (50mM PBS, 200mMNaCl, adjust pH to 6.0) to fully resuspend the bacteria, then put the centrifuge tube in an ice bath, put it in the ultrasonic cell disruptor, the ultrasonic disruption conditions are: working time 1s, stop Time is 2s, totaling 18min. The obtained crushed liquid was centrifuged at low temperature and high speed, and centrifuged at 4°C and 10,000 rpm for 30 minutes to...
Embodiment 3
[0045] Example 3: Determination of the catalytic efficiency of the mutant enzyme of Dorea sp. DPEase.
[0046] The present invention refers to the method for measuring the activity of D-psicose 3-epimerase. The mutant pure enzyme is subjected to a catalytic reaction under standard reverse conditions, and the synthetic amount of D-psicose is detected by HPLC, and the mutant is calculated Enzyme activity. The enzyme activity of wild-type Doreasp.DPEase is defined as the relative enzyme activity 100%. It was found that the specific enzyme activity of the mutant A38E / G105A was 1113.7 U / mg, which was an increase of 38.6% compared to the original enzyme specific enzyme activity of 803.5 U / mg.
[0047] Table 3 Comparison of relative enzyme activity between wild enzyme and mutant enzyme under optimal reaction conditions
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com