Nitrile hydratase mutant, genetically engineered bacteria containing mutant and application of mutant
A technology of nitrile hydratase and mutants, applied in the field of enzyme engineering, can solve the problems of low thermal stability and achieve good enzymatic properties, good product tolerance, and improved thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Construction of recombinant E. coli
[0055] (1) Construction of mutant αL6T / A19V / F126Y-βM46K / G47N / E108R / S212Y:
[0056] The nitrile hydratase mutant αL6T / A19V / F126Y-βM46K / E108R / S212Y gene was synthesized by chemical synthesis, and the gene was cloned at the NdeI and Bpu10I restriction sites of the pET24a plasmid, completed by General Biological Systems (Anhui) Co., Ltd. , Obtained pET24a-αL6T / A19V / F126Y-βM46K / E108R / S212Y recombinant plasmid. Using pET24a-αL6T / A19V / F126Y-βM46K / E108R / S212Y as a template, PCR was performed under the conditions shown in Table 1. The sequence information of the upstream primers used is shown in SEQ ID NO.9, and the sequence information of the downstream primers used is shown in SEQ ID NO. Shown in .10. The PCR product was transformed into E. coli JM109 to obtain the recombinant plasmid pET24a-αL6T / A19V / F126Y-βM46K / G47N / E108R / S212Y carrying the gene encoding the mutant. The recombinant plasmid pET24a-αL6T / A19V / F126Y-βM46K / G47N / E108R / ...
Embodiment 2
[0066] Example 2 Expression of Nitrile Hydratase
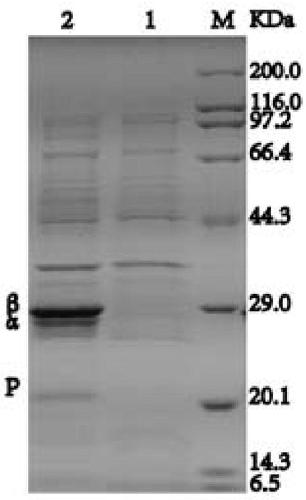
[0067] The BL21 / pET24a-αL6T / A19V / F126Y-βM46K / G47N / E108R / S212Y recombinant E. coli was inoculated into 5 mL of LB medium with a kanamycin concentration of 100 μg / mL, and cultured overnight at 37° C. with 200 r / min shaking. The above-mentioned overnight culture was inoculated into LB medium containing 100μg / mL kanamycin at the inoculum amount of 1%, and cultured with shaking at 37°C and 200r / min to the OD of the bacterial solution 600 To 0.6-0.8, add IPTG to a final concentration of 0.4mmol / L, induce culture at 20°C for 16-20h, collect the bacteria and ultrasonically break, analyze and identify the expression level of nitrile hydratase recombinant protein by Tris-tricine SDS-PAGE method, the results are as follows figure 1 Shown. After sonication and centrifugation at 12000 rpm for 60 minutes, the protein was purified with affinity chromatography column Strep Trap FF. The specific enzyme activity of the recombinant nitrile hydratase...
Embodiment 3
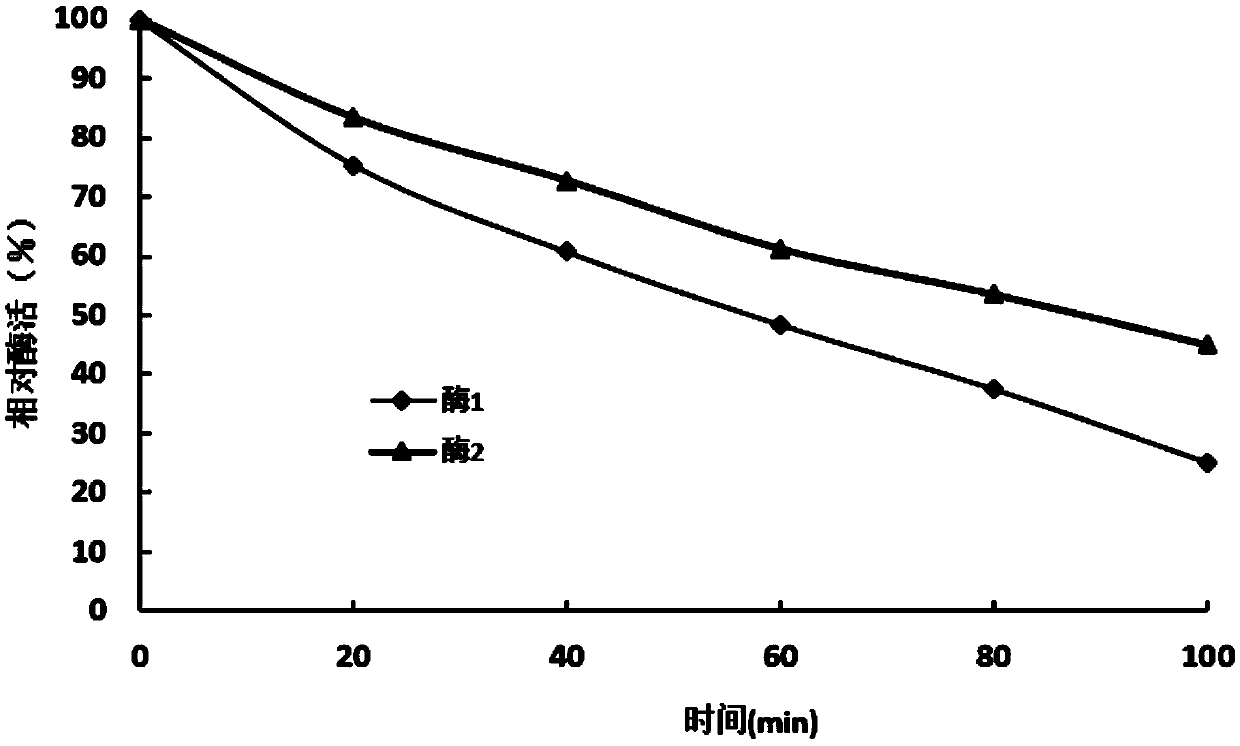
[0068] Example 3 Determination of thermal stability
[0069] In 500μL buffer reaction system (20mmol / L Na 2 HPO 4 , 280mmol / L NaCl and 6mmol / L KCl) was added 0.5mg / ml of the purified mutant enzyme in Example 1 10μL, stored in a metal bath at 50°C, and sampled every 20 minutes to determine the residual enzyme activity.
[0070] Such as figure 2 As shown, it was found that after the mutant was treated at 50°C for 80 minutes, the remaining enzyme activity of the mutant enzyme increased from 37% of the control (residual enzyme activity 333U / mg) to 53% (remaining enzyme activity 424U / mg); at 50°C After 100 minutes of treatment, the relative enzyme activity of the mutant enzyme increased from 24% of the control (remaining enzyme activity 216 U / mg) to 45% (remaining enzyme activity 360 U / mg). The thermal stability of the mutant is significantly improved.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com