Nicotinamide ribokinase mutant and application thereof
A technology of nicotinamide ribose and ribokinase, which is applied in the field of biological enzyme engineering, can solve the problems of less research and development, limited application, etc., and achieves the effects of mild reaction conditions, stable energy circulation system, and broad industrial application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Construction of Prokaryotic Expression System:
[0024] The NrK gene fragment was synthesized by Changzhou Jiyu Biotechnology Co., Ltd. and recombined into the PUC57 vector. After double digestion with restriction enzymes NdeI and HindIII (purchased from New England Biolabs, NEB) at 37°C for 4 hours, 1% agarose gel electrophoresis was used for separation and gel-cutting recovery (gel recovery kit purchased from Tiangen) Biochemical Technology (Beijing) Co., Ltd.). Subsequently, it was ligated with the expression vector pET29a(+) (Novagen), which was digested with the same double restriction enzymes, at 16°C overnight under the action of T4 DNA ligase (purchased from Takara). The ligation solution was transformed into Top10 competent cells (purchased from Tiangen Biochemical Technology (Beijing) Co., Ltd.), and colony PCR screening and sequencing were carried out to verify the positive recombinant plasmid NrK-pET29a(+).
[0025] The positive recombinant plasmid Nr...
Embodiment 2
[0027] Example 2 Enzyme shake flask fermentation to prepare enzyme freeze-dried powder:
[0028] The above-constructed expression strains NrK-pET29a(+) / BL21(DE3), PPK2-pET29a(+) / BL21(DE3), in 5ml LB liquid medium with a final concentration of 30μg / ml kanamycin sulfate [ 10g / l Tryptone (OXIOD), 5g / l Yeast Powder (OXIOD), 10g / l Sodium Chloride (Reagents of Chinese Medicines)] After shaking culture overnight at 37℃ and 200rpm, inoculate at the ratio of 1% (V / V) It was cultured in 500 ml LB liquid medium containing a final concentration of 30 μg / ml kanamycin sulfate at 37° C. and 200 rpm with shaking. When the OD600 is between 0.8-1.0, add the inducer IPTG (isopropyl-β-D-thiogalactoside, IPTG) with a final concentration of 0.1 mM, and induce overnight at 30°C. The cells were collected by centrifugation at 4°C and 8000rpm, then suspended in 50mM pH7.0 sodium phosphate buffer, ultrasonically disrupted (200W, 3s / 5s, 20min), centrifuged at 4°C, 12000rpm for 20min, and the supernatant wa...
Embodiment 3
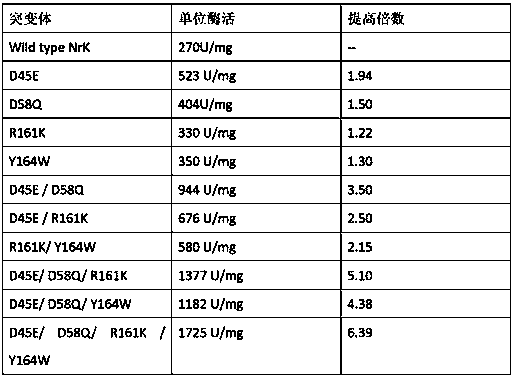
[0029] Example 3 Construction and screening of mutants:
[0030] Mutant construction: The method of homology modeling is used to simulate the three-dimensional structure of NrK, and the sites that may be related to catalysis and substrate binding are predicted using the principle of molecular docking and lowest energy, which are initially identified as D45, D58, R161 , Y164 four sites. Subsequently, using the NrK-pET29a(+) recombinant plasmid as a template, NNK saturation mutations were performed on these four sites respectively (refer to Stratagene's QuikChange® Site-Directed Mutagenesis Kit operating instructions for specific mutation operations). 45 mutation Forward primer: GATGATTTTTATAAACCGNNKAGCGAAATTCCGATTAACG, reverse primer: CGTTAATCGGAATTTCGCTMNNCGGTTTATAAAAATCATC; 58-point mutant forward primer: CGAAAAATATGGCGTGGCGNNKTGGGATTGCCCGGAAGCG, reverse primer: CGCTTCCGGGCAATCCCAMNNCGCCACGCCATATTTTTCG; 161-point mutant forward primer: GCCGCCGCCGCCATGCGNNKGCGGGCTATAAAACCCTGGAAG,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com