Method for increasing lipase expression through glycosylation modification as well as mutant enzyme and application thereof
A lipase and Rhizopus oryzae lipase technology, applied in the field of enzyme engineering, can solve the problems of low expression and enzyme activity, and achieve the effect of improving enzyme activity and secretion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Introduction of N-glycosylated mutant enzymes and construction of genetic engineering
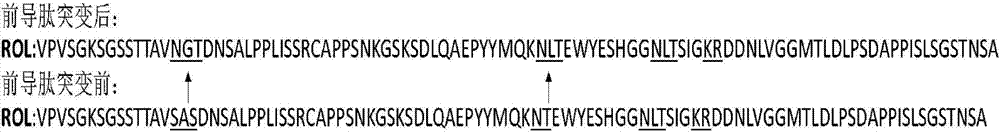
[0034] In the present invention, three kinds of N-glycosylation site mutants are designed in the leader peptide of Rhizopus oryzae lipase ROL, that is, SAS and NT amino acids are transformed into N-glycosylation sites NGT and NLT respectively, and the mutant enzymes are named as proROLA (amino acid sequence as shown in SEQ ID NO.1) and proROLB (amino acid sequence as shown in SEQ ID NO.2); naming of mutant enzymes that simultaneously transform SAS and NT into N-glycosylation sites It is proROLAB (the amino acid sequence is shown in SEQ ID NO.3). The specific mutation method of the leader peptide is as follows: figure 1 shown.
[0035] The specific construction method is as follows:
[0036] (1) With the carrier pPIC9K-proROL, pGAPZα of the Aspergillus oryzae lipase gene proROL containing the amino acid sequence shown in SEQ ID NO.4 as a template, simultaneously use rest...
Embodiment 2
[0041] Embodiment 2: the growth comparison of the genetically engineered bacteria expressing mutant enzyme and wild enzyme
[0042] Using GS115 / pGAPZα-proROL as a control, the growth of genetically engineered bacteria expressing the lipase mutant of Rhizopus oryzae was compared.
[0043] Shake flask fermentation conditions: Streak positive transformants on YPD-G418 plate ((w / v): yeast extract 1%, glucose 2%, agar powder 2%, tryptone 2%, G4180.025%), culture at 30°C 3d, pick a single clone and inoculate it into 100mLYPD liquid medium ((w / v): yeast extract 1%, glucose 2%, tryptone 2%), 30°C 200rpm·min -1 Shake flask culture, sampling every 12h or 24h.
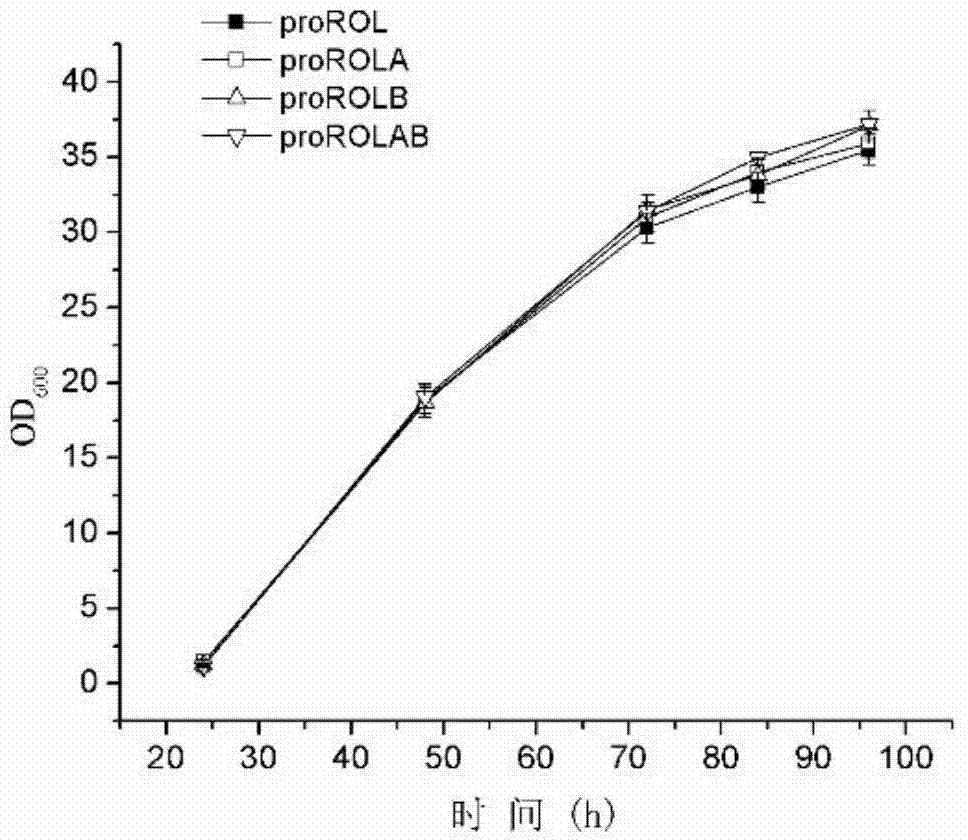
[0044] Strain growth curve as figure 2 shown. The results showed that the growth of the mutant strains was similar to that of the control. The strains grew rapidly within 48 hours, and after 72 hours, the growth of each strain became slow and tended to be stable. It shows that the introduction of glycosylation site into the ...
Embodiment 3
[0045] Example 3: Effect of N-glycosylation on the Secretion Level of Rhizopus oryzae Lipase
[0046] The present invention compares the lipase secretion situation of genetically engineered bacteria expressing mutant enzymes and wild enzymes.
[0047] Each genetically engineered bacteria was fermented and cultured, and the specific culture conditions were: Streak the positive transformants on the YPD-G418 plate, culture at 30°C for 3 days, pick a single clone and inoculate it into 100mL YPD liquid medium, 30°C, 200rpm·min -1 Shake flask culture, sampling every 12h or 24h.
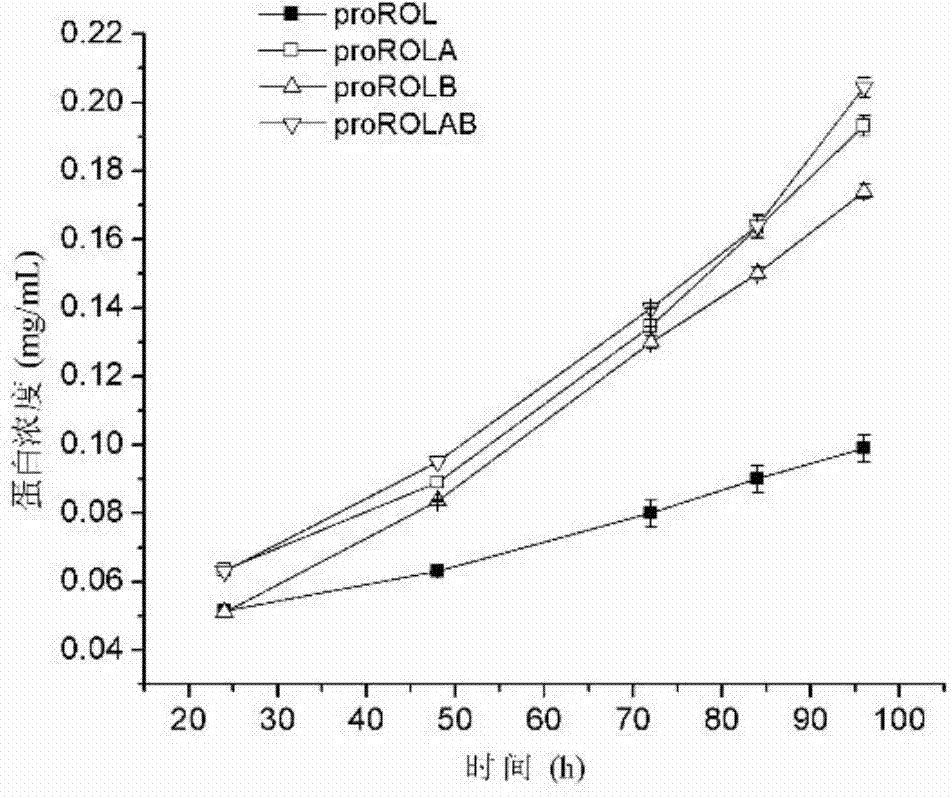
[0048] The result is as image 3 As shown, the protein concentration of rhizopus oryzae lipase proROL without glycosylation sites was the lowest, while the extracellular protein concentration of rhizopus oryzae lipase proROLAB with two glycosylation sites was slightly higher than that with only one sugar The lipases proROLA and proROLB at the sylation site were preliminarily judged that the introduced gly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com