Method for modifying flavonoid glycoside compounds with galactosy transferase
A technology of flavonoids and compounds, applied in the field of biopharmaceuticals, can solve the problems of low solubility of natural flavonoid glycoside compounds, achieve the effects of improving bioavailability, easy acquisition, and improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] Preparation of enzyme extract:
[0040]Construct the expression plasmid containing β-1,4-galactosyltransferase gene, transfer it into Escherichia coli BL21 (DE3) strain, induce the expression of the target protein with isopropylthiogalactopyranoside (IPTG), sonicate the cells, After Ni column affinity purification and ultrafiltration concentration, the enzyme extract with high purity is obtained. The molecular weight and purity of the target protein were tested by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the concentration of the enzyme extract was determined by BIOFORD method. The obtained enzyme extract was added with 50% glycerol in the total volume of the enzyme extract and DTT with a final molar concentration of 0.01mmol / L, and stored at -20°C for future use. Avoid repeated freezing and thawing to avoid the decrease in enzyme activity.
[0041] This method is applicable to natural flavonoid glycoside compounds, especially flavonoid ...
Embodiment 1
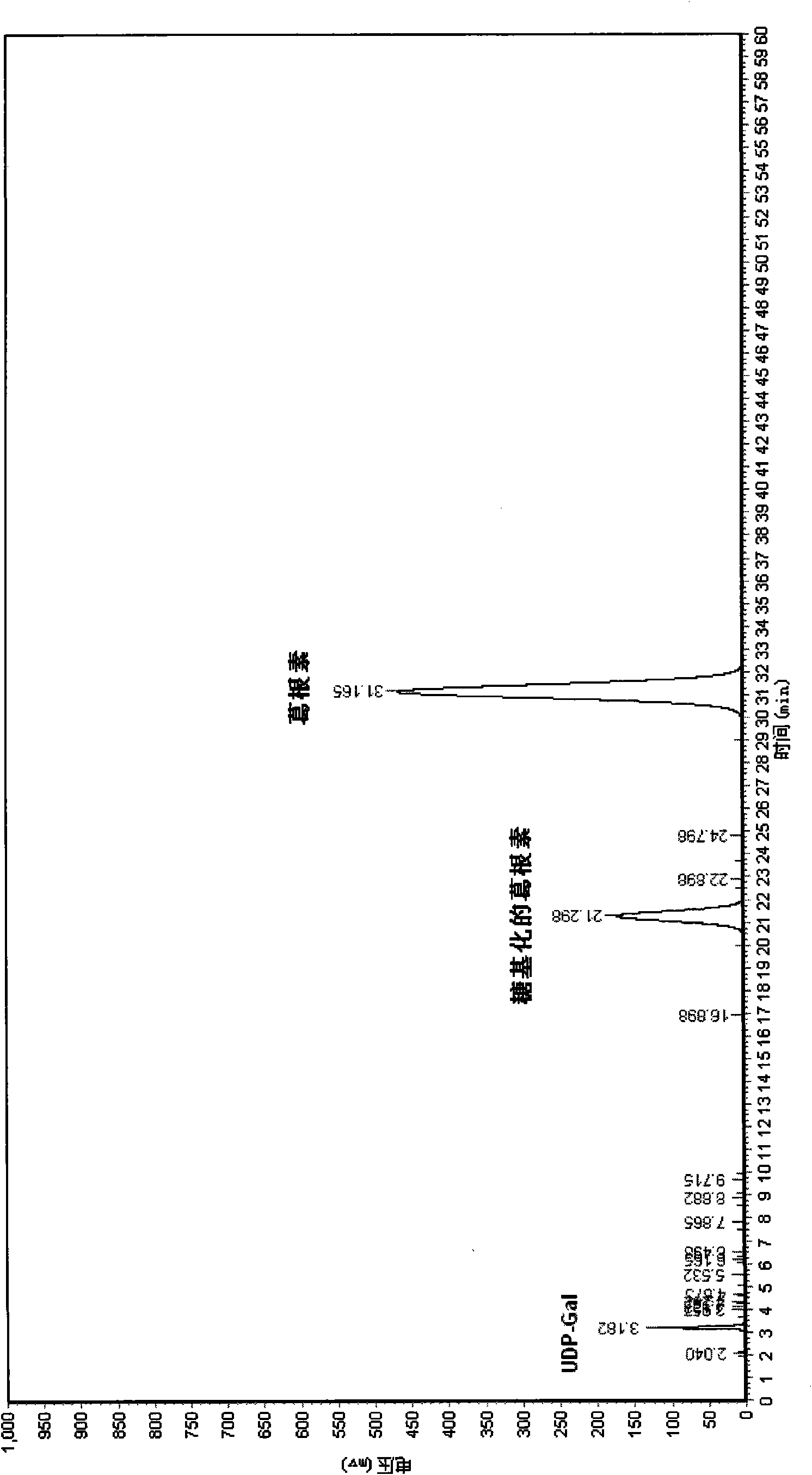
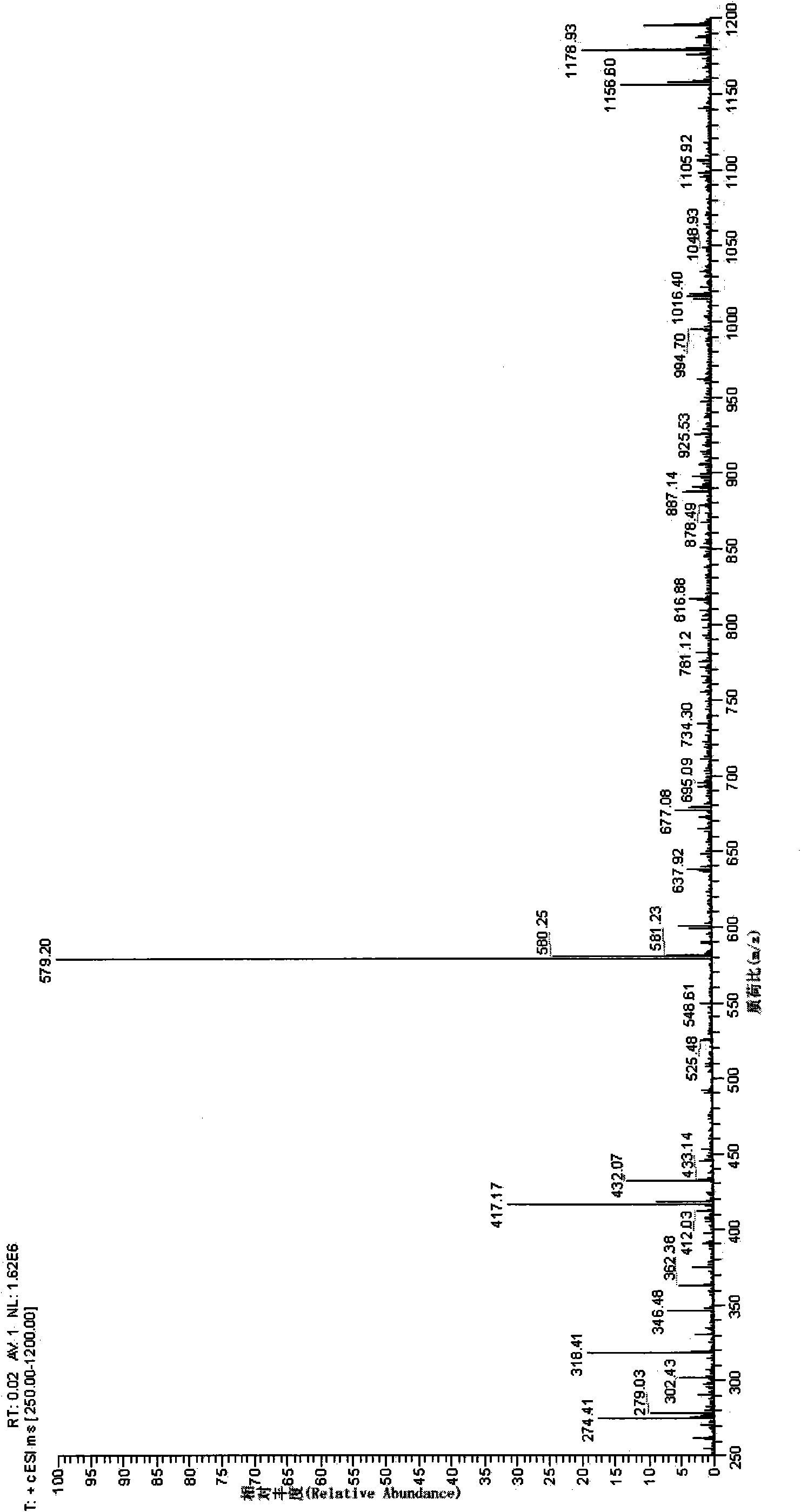
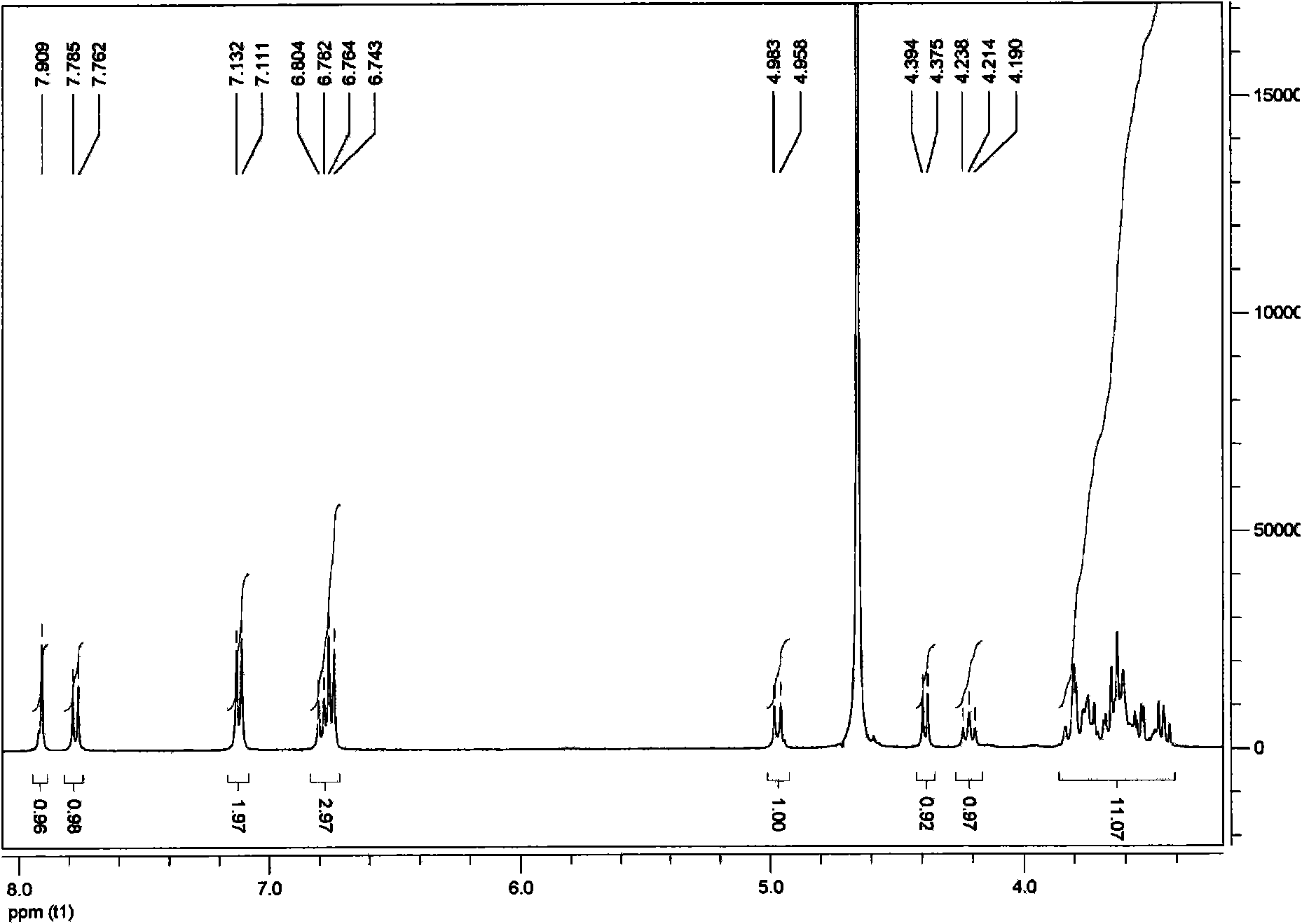
[0042] Example 1: Synthesis of Glycosylated Puerarin
[0043] Prepare fresh enzymatic reaction buffer according to the method in the above embodiment, weigh Hepes 0.952g, BSA 0.4g, MnCl 2 4H 2 O 0.00475g, after being fully dissolved in distilled water, dilute to 200mL. Then prepare 9.12mg / mL puerarin solution and 4.00mg / mL UDP-galactose solution with enzymatic reaction buffer respectively.
[0044] Put 5 mL of puerarin solution and 5 mL of UDP-galactose solution into the reaction vessel, the molar final concentration ratio is 10:3; then add 200 μL of enzyme extract (enzyme extract is prepared according to the method in the above embodiment); Make up the volume of reaction buffer to 50mL and mix gently. React at 37°C for 30 minutes. After the reaction was completed, the reaction vessel was placed in a boiling water bath for 5 minutes to remove the protein in the reaction solution. After cooling to room temperature, centrifuge at 12,000 rpm for 10 minutes, and discard the p...
Embodiment 2
[0051] Embodiment 2: the synthesis of glycosylated naringin
[0052] Prepare fresh enzymatic reaction buffer according to the method in the above embodiment, weigh Hepes 0.952g, BSA0.4g, MnCl 2 4H 2 O 0.00475g, after being fully dissolved in distilled water, dilute to 200mL. Then prepare 1.45mg / mL naringin solution and 29.3mg / L UDP-galactose solution with enzymatic reaction buffer respectively.
[0053] Add 42mL of naringin solution and 5mL of UDP-galactose solution into the reaction vessel, and the molar final concentration ratio is 7:16; then add 200 μL of enzyme extract (enzyme extract is prepared according to the method in the above embodiment); Accelerate the reaction buffer to make up the volume to 50mL, and mix gently. React at 37°C for 30 minutes. After the reaction was completed, the reaction vessel was placed in a boiling water bath for 10 minutes to remove the protein in the reaction solution. After cooling to room temperature, centrifuge at 10,000 rpm for 10 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com