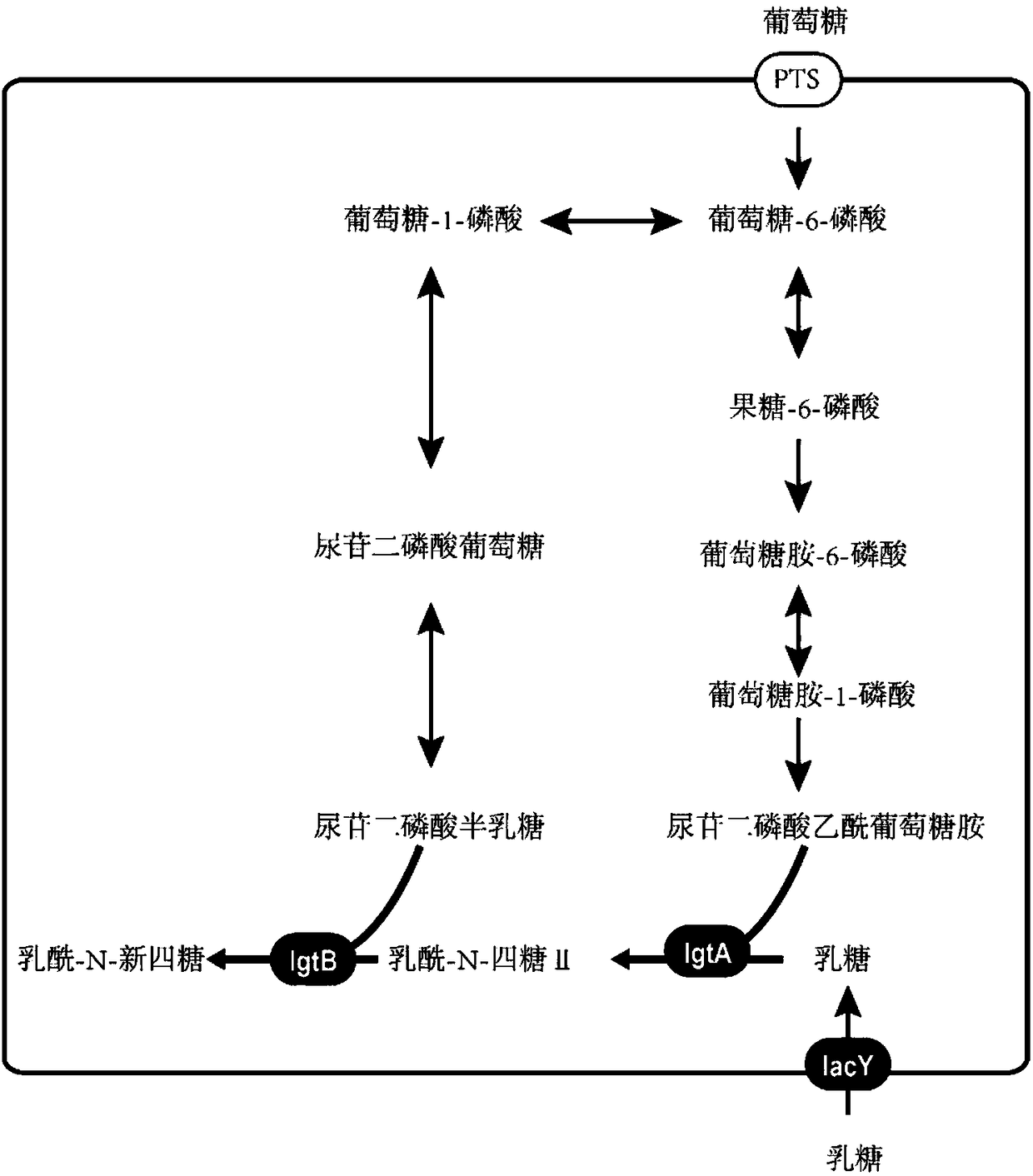
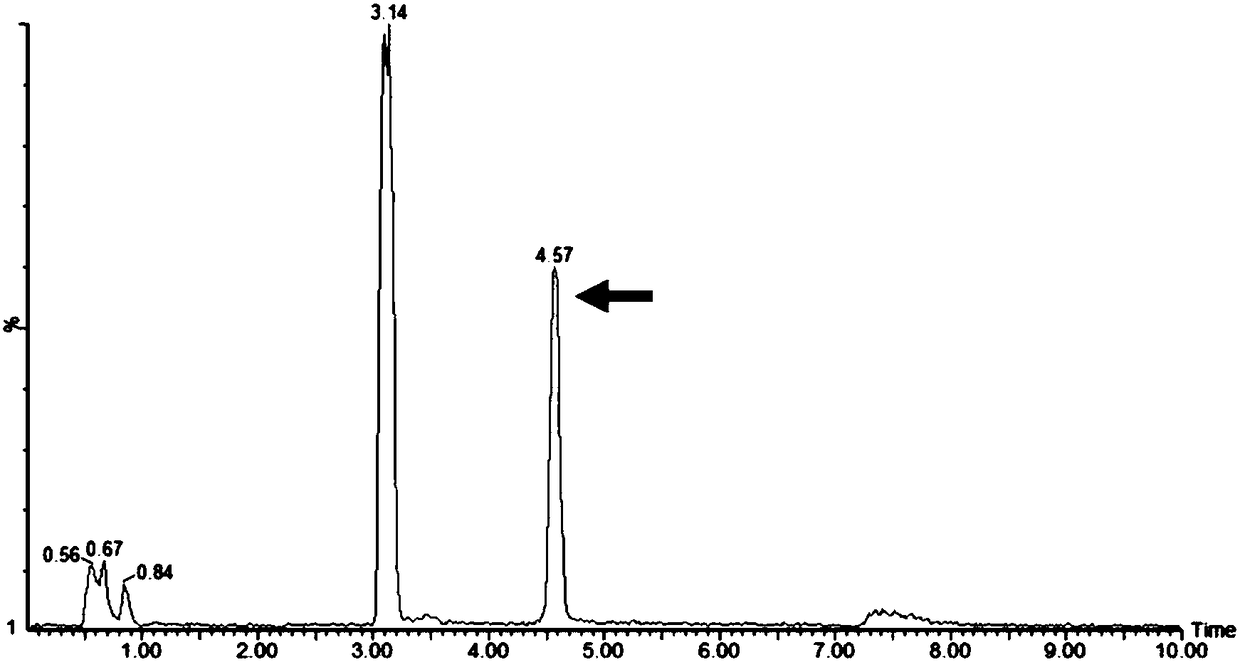
Recombinant bacillus subtilis for synthesizing lactyl-N-neotetraose and construction method and application of recombinant bacillus subtilis
A technology of Bacillus subtilis and construction method, which is applied in the direction of recombinant DNA technology, bacteria, and the introduction of foreign genetic material using vectors, etc. It can solve the problems of inapplicability of toxic solvents, low synthesis yield, cumbersome protection, deprotection operation, separation and purification, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Construction of recombinant fragments
[0025] Using the Bacillus subtilis (Bacillus subtilis 168) genome as a template, according to the amyE gene (Gene ID: 938356) published on NCBI, the primers for the homology arms on both sides were designed, and the sequences were SEQ ID NO: 1 and SEQ ID NO: 2 Left homology arm primer:
[0026] amyE-1F:5'-TATTCCGTATGTCAAGTGGCTGCGGTTTAT-3' (SEQ ID NO: 1),
[0027] amyE-1R:5'- AATTGTTATCCGCTCTCTTGACACTCCTTATTTGA TTTTTTGAAGACTTACTTCGG-3' (SEQ ID NO: 2),
[0028] The sequences are respectively the right homology arm primers of SEQ ID NO:3 and SEQ ID NO:4:
[0029] amyE-2F:5'- CTTAAGGGCAAGGCTAGACGGGACTTA -3' (SEQ ID NO: 3),
[0030] amyE-2R:5'-GGCACACCGATGTACACGTCATC-3' (SEQ ID NO:4),
[0031] Use the above primers to amplify the homology arm gene sequences on both sides of amyE from the Bacillus subtilis genome; use the plasmid pP43NMK as a template to design primers whose sequences are SEQ ID NO:5 and SEQ ID NO:6:...
Embodiment 2
[0044] Example 2 Construction of recombinant plasmids
[0045] According to the β-1,3-N-glucosaminyltransferase gene lgtA (Gene ID: 904226) in Neisseria meningitidis MC58 published on NCBI, after codon optimization by Bacillus subtilis, synthesize as the gene sequence shown in SEQ ID NO:14, CTGCCGGAAGAAGATTTTGAAAGAGCAAGACGCTTTCTTTACCAATGTTTTAAACGCACAGATACATTACCGGCTGGCGCCTGGCTGGATTTTGCAGCGGATGGAAGAATGAGACGCTTATTTACACTGCGCCAGTACTTTGGAATCCTGCATAGACTGCTGAAAAACCGCTAA (SEQ ID NO: 14)
[0046] The primers whose sequences are respectively designed as SEQ ID NO:15 and SEQ ID NO:16:
[0047] lgtA-1F:5'- ATGCCGTCTGAAGCTTTTAG ACGC-3' (SEQ ID NO: 15),
[0048] lgtA-1R:5'- TTAGCGGTTTTTCAGCAGTC TATGCAGGATT-3' (SEQ ID NO: 16),
[0049] Using the above primers, the lgtA gene fragment was amplified using the synthetic β-1,3-N-glucosaminyltransferase gene (SEQ ID NO: 14) as a template.
[0050] According to the β-1,4-galactosyltransferase gene lgtB (Gene ID: 904227) in Neisseria mening...
Embodiment 3
[0059] Example 3 Construction of Recombinant PZL Fragment Bacillus subtilis
[0060] The constructed recombinant fragment PZL was transformed into Bacillus subtilis competent cells (Bacillus subtilis168), the amount of recombinant fragment added was 100-300ng, electroporation conditions: voltage 2.5kV, electroporation reagent 5ms, 37°C recovery for 5h, and then coated with Bleomyces Incubate at 37°C for 24 hours. Using lacY-F and lacY-R primers (shown in SEQ ID NO:9 and SEQ ID NO:10 respectively) to select transformants for colony PCR verification, a 1295bp band appeared, and it was verified that the recombinant Bacillus subtilis mA0 was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com