Indole compounds and preparation method and application thereof as drug-resistant bacteria resistant drugs
A compound and indole technology, applied in the field of chemical and compound medicine, can solve problems such as slow progress and inability to treat drug-resistant bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
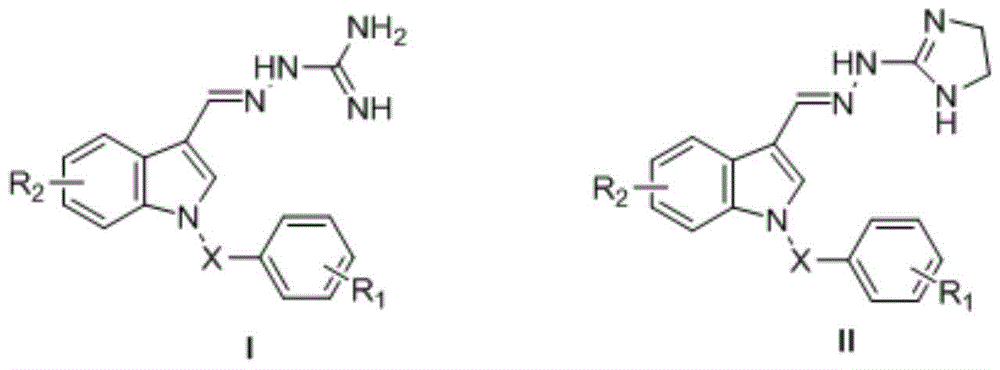
[0168] Embodiment 1 general formula III, the preparation of I compound
[0169]
[0170] Preparation of intermediate 1-(3- or 4-substituted benzyl)-3-formyl-5(or 6)-methoxycarbonylindole 3 in general formula V
[0171]
[0172] Preparation of 3-formyl-5(or 6)-methoxycarbonylindole (2)
[0173] 5 (or 6)-methoxycarbonylindole (1) and phosphorus oxychloride (POCl 3 ) Vilsmeier-Hack reaction occurs under the condition of dry N,N-dimethylsulfoxide to generate 3-formyl-5 (or 6)-methoxycarbonylindole (2)
[0174] Preparation of 3-formyl-5 (or 6)-methoxycarbonylindole (2) manipulation example
[0175] Preparation of 3-formyl-5-methoxycarbonylindole
[0176]Under nitrogen protection, 5.00 g (28.57 mmol) of methyl indole-5-carboxylate was added to 20 mL of ultra-dry DMF, cooled to 0°C, and then 3.62 mL (38.86 mol) of phosphorus oxychloride was slowly added dropwise. After reacting at 0°C for 10 min, react at room temperature for 3 h. After cooling the reaction liquid to 0°C, ...
Embodiment 2
[0236] Embodiment two general formula IV, the preparation of II compound
[0237] Preparation of target compound Ⅳ
[0238] Intermediate (3) or (4) or (8) respectively reacts with 4,5-dihydroimidazole-2-hydrazine hydrogen bromide VI in dry methanol to reflux the target compound IV;
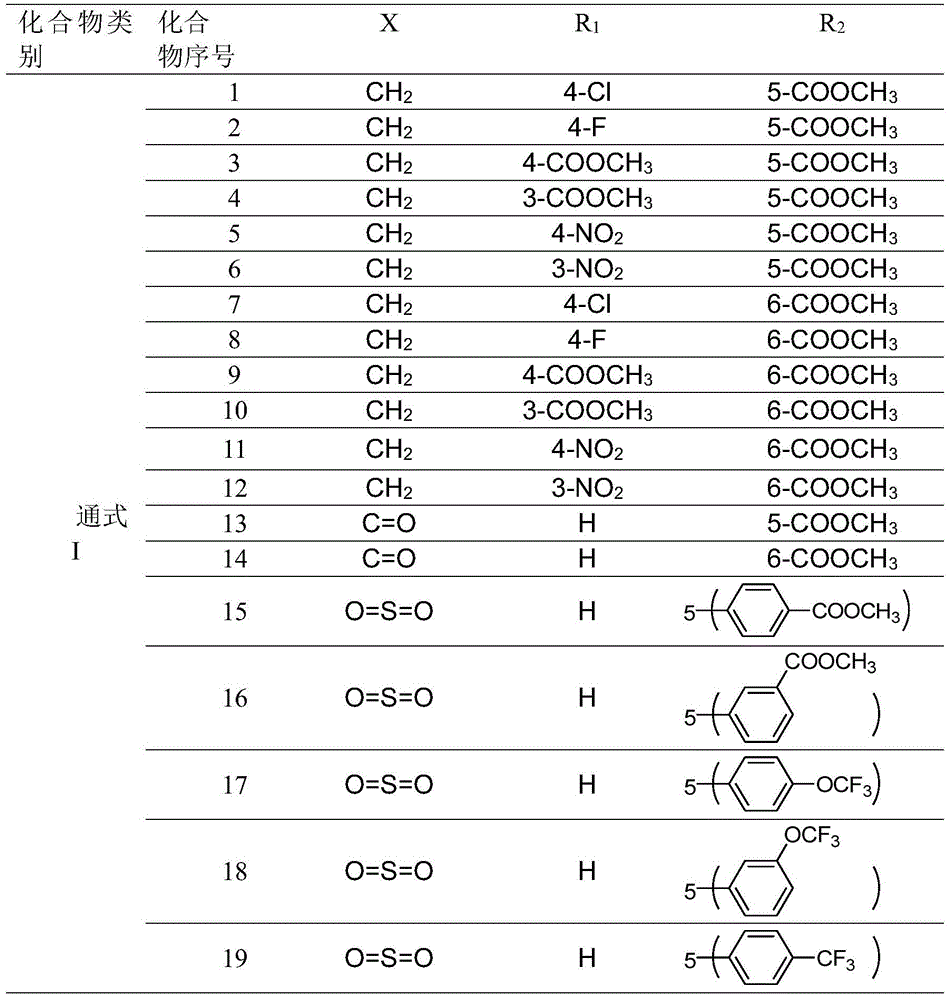
[0239] Preparation of 1-p-chlorobenzyl-5-methoxycarbonyl-3-indolecarbaldehyde, (4,5-dihydro-1H-imidazol-2-yl)hydrazonone hydrogen bromide salt (compound 61 in Table 8) manipulation instance
[0240] 0.05 g (0.15 mmol) of 1-p-chlorobenzyl-3-formyl-6-methoxycarbonylindole was added to 2 mL of anhydrous methanol, and then 4,5-dihydroimidazole-2-hydrazine was brominated 0.03 g (0.15 mmol) of hydrogen was added to the reaction solution, and heated to 75°C for reaction. After the reaction, the reaction solution was cooled to room temperature, and diethyl ether was added to stand still to precipitate a solid, which was filtered under reduced pressure, and the filter cake was washed with diethyl ether ...
Embodiment 3
[0250] Embodiment 3 The preparation of compound sterile powder injection of the present invention
[0251] 1. Prescription:
[0252] Prescription 1:
[0253] Any one of compound III or IV 250g (calculated as compound)
[0254] A total of 1000 sticks were prepared
[0255] Prescription 2:
[0256] Any one of compound III or IV 500g (calculated as compound)
[0257] A total of 1000 sticks were prepared
[0258] Prescription 3:
[0259] Any one of compound III or IV 1000g (calculated as compound)
[0260] A total of 1000 sticks were prepared
[0261] Prescription 4:
[0262] Any one of compound III or IV 2000g (calculated as compound)
[0263] A total of 1000 sticks were prepared
[0264] 2. Preparation process:
[0265] (1) Aseptically process the antibiotic glass bottles, rubber stoppers, etc. used for the preparation;
[0266] (2) Weigh the raw materials according to the prescription (feeding after conversion), put the sterile powder in the filling machine for subp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com