Method for synthesizing avibactam intermediate compound
A synthesis method and compound technology, which can be used in organic chemistry, bulk chemical production, etc., can solve the problems of expensive raw materials, unfriendly environment, expensive process conditions, etc., and achieve the effect of mild reaction conditions, no heavy metal pollution, and environmental friendliness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
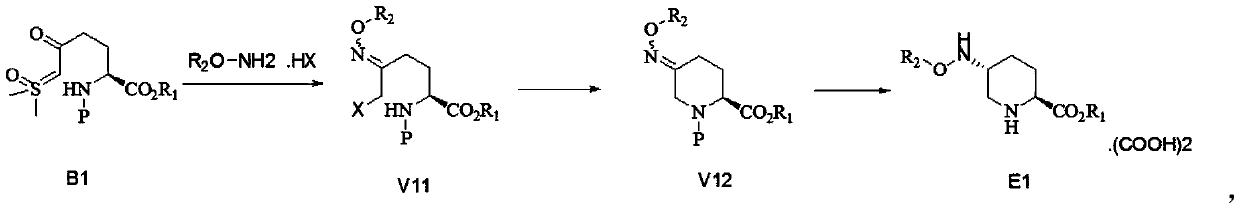
[0040] Embodiment one: E1-2 preparation
[0041]
[0042] 1. Preparation of intermediate V11-2:
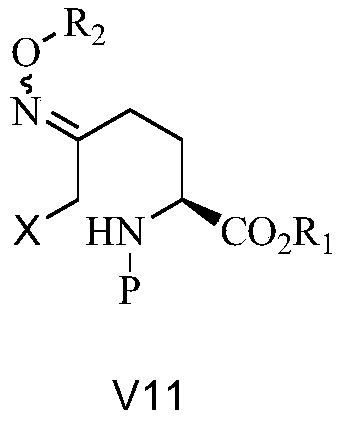
[0043] Under nitrogen protection, add 159kg of B1-2 and 776kg of ethyl acetate into a 1500 reactor, under nitrogen protection, add 78kg of O-benzylhydroxylamine hydrobromide, heat up to 50°C and stir for 2 hours. Cool down to room temperature, wash the reaction solution with 300kg of water, and then concentrate under reduced pressure to obtain V11-2. 1H NMR (400MHz, CDCl3) δ7.232 (w, 10H, PhH); 5.177~4.966 (m, 5H, 2PhCH2, NH); 4.272(w, 1H, NCH); 3.843~3.781(m, 2H, BrCH2); 2.405~2.268(m, 2H, NCCH2); 2.036(w, 1H, CH2); 1.866(w, 1H, CH2).
[0044] 1H NMR (400MHz, CDCl3, D2O) δ7.232(w, 10H, PhH), 5.276~4.949(m, 4H, 2PhCH2); 4.257(w, 1H, NCH); 3.841~3.766(m, 2H, BrCH2) ;2.396~2.268(m,2H,NCCH2);2.034(w,1H,CH2);1.843(w,1H,CH2).
[0045] 2. Preparation of intermediate V12-2:
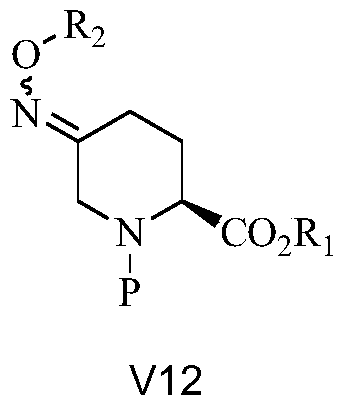
[0046] Add V11-2 in the previous step to 500kg DMF, then add 85kg sodium bis(trimethylsilyl)amide, and rea...
Embodiment 2
[0050] Embodiment 2: Preparation of E1-1
[0051]
[0052] 1. Preparation of intermediate V11-3. Preparation process is the same as embodiment 1.
[0053]V11-3: 1H NMR (400MHz, CDCl3) δ7.268(w, 5H, ArH); 5.084~5.020(m, 3H, PhCH2O, NH); 4.217(w, 1H, COCH); 4.152~4.016(m, 2H, CH3CH2O); 3.839~3.576(m, 2H, ICH2); 2.385~1.787(m, 4H, 2CH2); 1.369(s, 9H, 3CH3); 1.176(q, 3H, J=8, CH3); 1H NMR(400MHz, CDCl3, D2O) δ7.268(w, 5H, ArH); 5.084~4.975(m, 2H, PhCH2O); 4.206(w, 1H, COCH); 4.135~4.015(m, 2H, CH3CH2O); 3.839~3.609(m,2H,ICH2); 2.400~1.850(m,4H,2CH2); 1.368(s, 9H,3CH3); 1.176(q,3H,J=8,CH3)
[0054] 2. Preparation of intermediate V12-1:
[0055] Preparation process is the same as embodiment 1.
[0056] V12-1 1H NMR (400MHz, CDCl3) δ7.281~7.216(m, 5H, PH); 5.007(s, 2H, PhO-CH2); 4.720~4.241(m, 2H, NCH2); 4.138~4.105(m ,2H,OCH2); 3.795(dd,1H,J1=15.2,J2=19.2,COCH); 2.774~1.941(m,4H,2CH2); 1.408~1.351(w,9H,3CH3);1.213~1.176(t ,J=6.4,3H,CH3)
[0057] 3. Preparation of intermed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com