Preparation methods of sodium avibactam and intermediate compound thereof
A kind of technology of avibactam sodium and compound, which is applied in the field of chemical pharmacy, can solve the problems of high risk of hydrogen reduction, unsuitability for industrial production, cumbersome reaction operation process, etc., so as to avoid hydrogenation catalytic operation, maximize application value and reduce safety risk effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
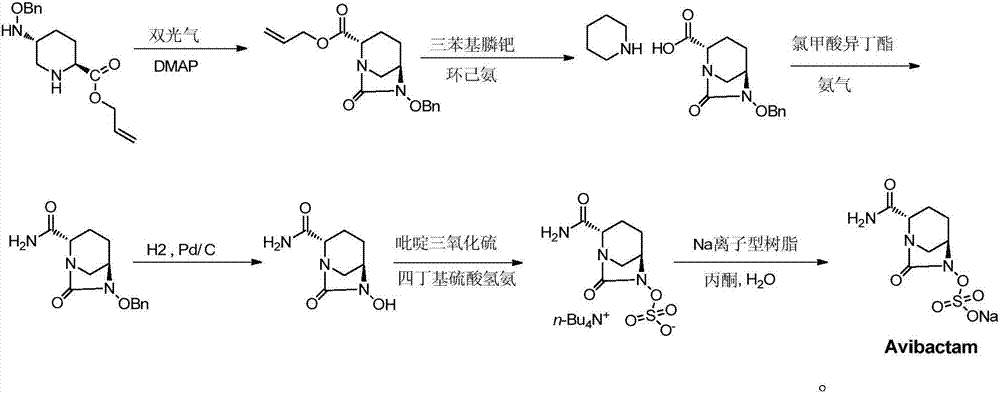
[0044](2S,5R)-6-Benzyloxy-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3,2,1]octane-2-carboxamide (Compound J) preparation: (refer to patent document CN103649051) at 20°C, (2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxamide (102g, 409mmol) and di Isopropylamine (76.2ml, 437.6mmol) and chlorobenzene (612ml) were mixed. 9-Perlenyl chloroformate (107.9 g, 417.2 mmol) was added to the reaction mixture as a solution in chlorobenzene (612 ml), and the mixture was stirred at 30°C until the reaction was complete. Carbonyldiimidazole (86.2 g, 531.7 mmol) was added and stirring continued until the reaction was deemed complete. Diethylamine (105.8ml, 1022.5mmol) was added and stirring continued until the reaction was deemed complete. Aqueous hydrochloric acid (640.0ml, 3N, 1920ml) was added and the mixture was cooled to 2°C. The solid was isolated by filtration, washed with water (2X, 200ml) and dried to afford the title compound (101g, 367.2mmol, 90%) as a white crystalline solid.
[0045] ...
Embodiment 2
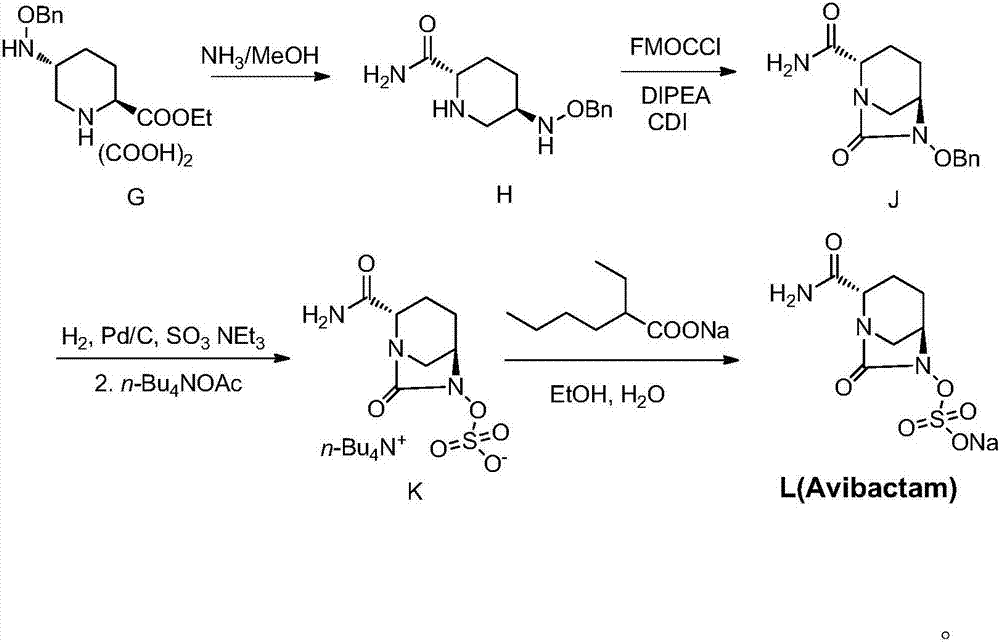
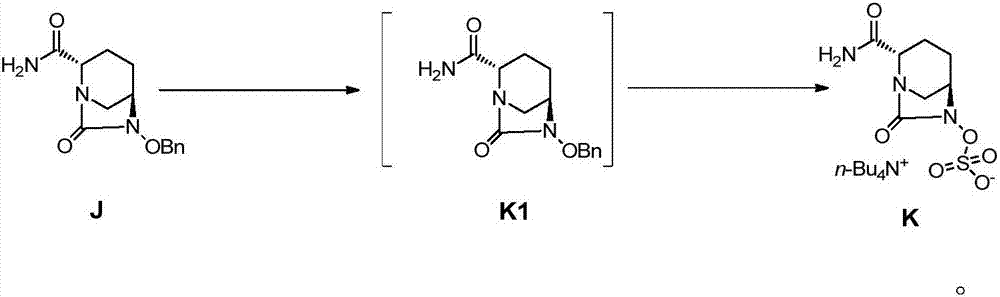
[0049] ({[(2S,5R)-2-carbamoyl-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3,2,1]-octyl-6- Base]oxy}sulfonyl)tetrabutylammonium salt (compound K): isopropanol (150ml) was added to the reaction flask, compound J (10.0g), palladium on carbon (1.0g), triethylamine (3.68g), ammonium formate (1.15g) and formic acid (3.34g), controlled temperature 30 ℃ ~ 35 ℃ reaction 2 ~ 3 hours, HPLC detection J after the reaction is complete, suction filtration, washing. Triethylamine (0.92 g) and trimethylamine sulfur trioxide (7.1 g) were added to the filtrate. The temperature is controlled at 30°C to 35°C, and the reaction is stirred for 3 to 4 hours. Add content and be 35% tetrabutylammonium acetate (16.5g) aqueous solution, stir and react at room temperature for 3 hours, the reaction solution is concentrated to 140g, dichloromethane (60g×2) is added to the concentrated reaction solution for extraction twice, after phase separation, the The organic phase was concentrated under vacuum at 40-50°C to o...
Embodiment 3
[0053] ({[(2S,5R)-2-carbamoyl-7-oxo-6-(sulfooxy)-1,6-diazabicyclo[3,2,1]-octyl-6- Base]oxy}sulfonyl)tetrabutylammonium salt (compound K) preparation: isopropanol (150ml) was added in the reaction flask, compound J (10.0g) was added, palladium hydroxide (1.0g), triethyl Amine (3.68g), ammonium formate (1.15g) and formic acid (3.34g) were reacted at a controlled temperature of 30°C to 35°C for 2 to 3 hours. After the reaction was detected by HPLC, the mixture was suction filtered and washed. Triethylamine (0.9 g) and trimethylamine sulfur trioxide (7.1 g) were added to the filtrate. The temperature is controlled at 30°C to 35°C, and the reaction is stirred for 3 to 4 hours. Add content and be 35% tetrabutylammonium acetate (11g) aqueous solution, stir and react at room temperature for 3 hours, the reaction solution is concentrated to 150g, dichloromethane (60g×2) is added to the concentrated reaction solution and extracted twice, after phase separation, the organic Concentrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com