A kind of avibactam intermediate, preparation method and application thereof
An intermediate and reaction technology, applied in the field of drug synthesis, can solve the problems of low yield, salt formation, cumbersome operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
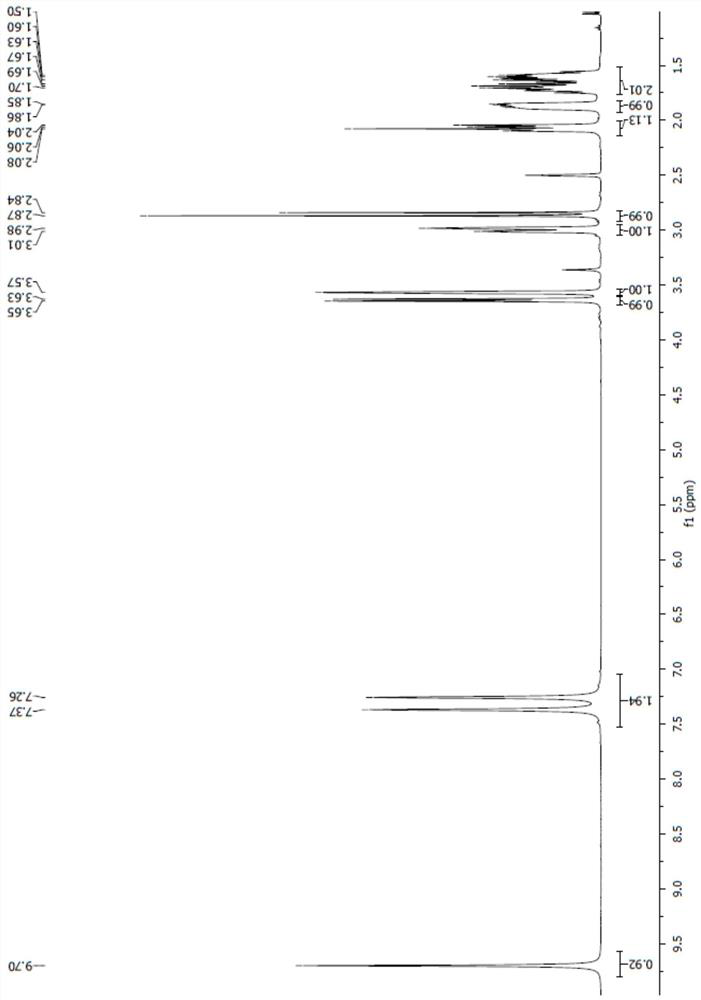
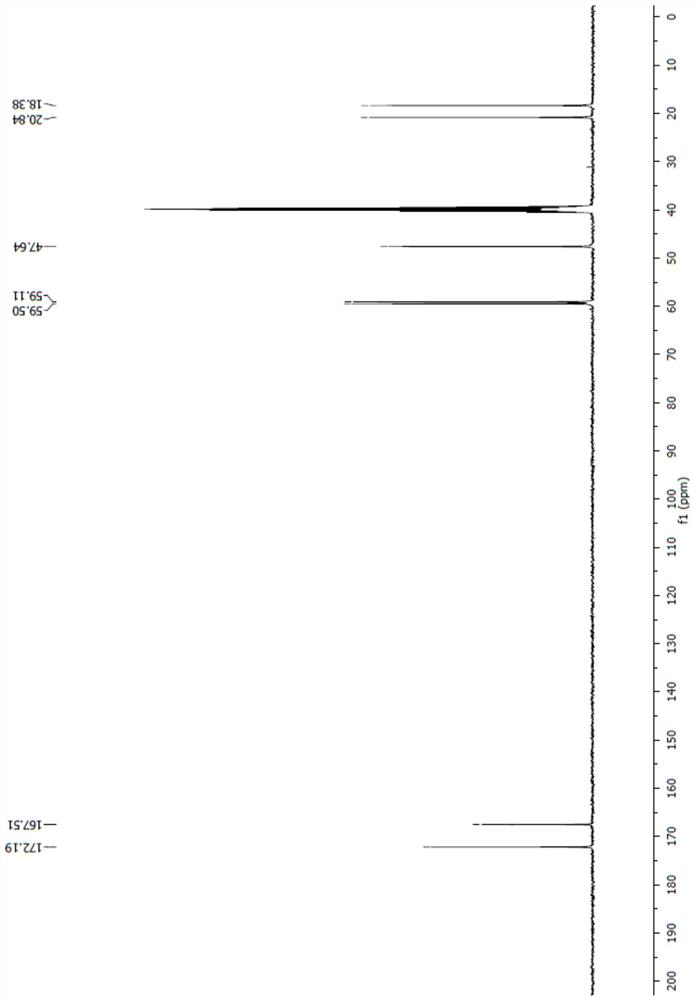
[0047] 5g (18mmol ), acetone 50ml, palladium carbon 0.5g, replaced with nitrogen at least 3 times, replaced with hydrogen 3 times, at 25 ~ 35 ℃, passed through hydrogen for 3 hours, TLC detected that the reaction was complete, replaced 3 times with nitrogen, filtered with The filter cake was rinsed with acetonitrile, the temperature of the filtrate was cooled to 0°C, 9.7 g (72 mmol) of benzyldimethylamine was added, and 4.2 g (36 mmol) of chlorosulfonic acid was added dropwise. Insulate for 2 hours, the reaction is complete, the reaction solution is decompressed to dryness, add 50ml of dichloromethane, 30ml of pure water, separate the phases, extract the water phase with 20ml of dichloromethane once, combine the organic phases, depressurize to dryness, add 20ml of isopropyl ether , a solid precipitated, suction filtered, rinsed with isopropyl ether, and dried at 35°C to obtain compound III ([(1R,2S,5R)-2-(aminocarbonyl)-7-oxo-1,6-di Azabicyclo[3.2.1]oct-6-yl]benzyldimethylamm...
Embodiment 2
[0050] Put 5g (18mmol) of compound I, 50ml acetone, and 0.5g palladium carbon into the reaction bottle, replace with nitrogen for at least 3 times, and replace with hydrogen for 3 times. Replaced with nitrogen 3 times, filtered with suction, washed the filter cake with acetone, cooled the filtrate to 0°C, added 9.7g (72mmol) of benzyldimethylamine, and added 3.5g (36mmol) of sulfuric acid dropwise. Keep warm for 2 hours, and the reaction is complete. The reaction solution was decompressed to dryness, added 50ml of dichloromethane, 30ml of pure water, separated the phases, extracted the aqueous phase once with 20ml of dichloromethane, combined the organic phases, decompressed to dryness, added 20ml of isopropyl ether, a solid precipitated, pumped Filter, rinse with isopropyl ether, and dry at 35°C to obtain 5.9g of compound III, with a yield of 83% and a content of >99%.
[0051] Put 5.0g of compound III into the reaction bottle, add 25ml of ethanol at 20-35°C, dissolve at 20-...
Embodiment 3
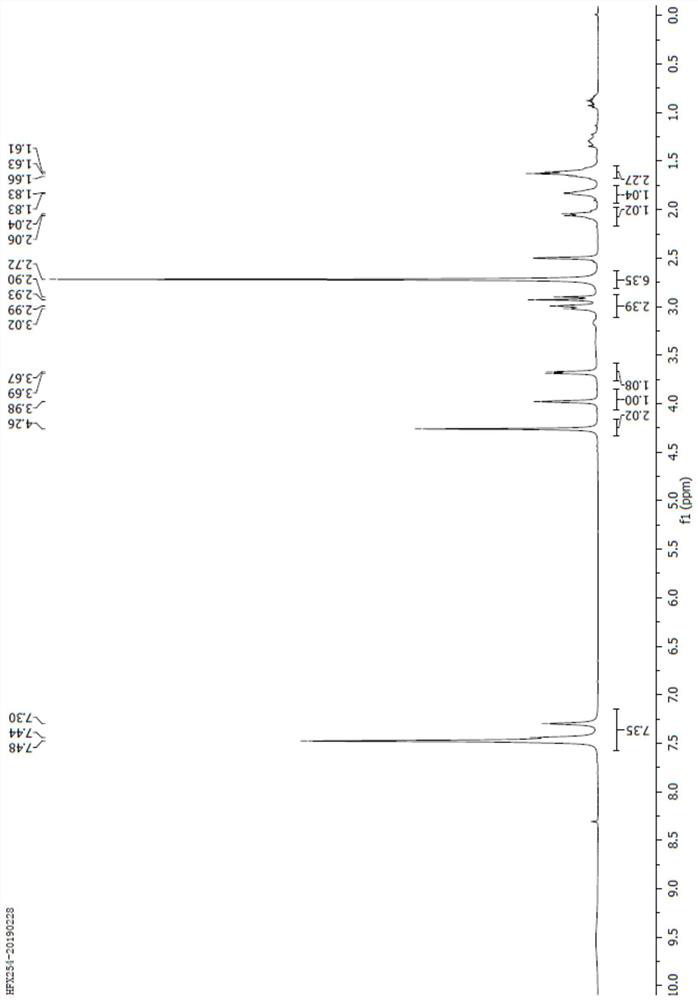
[0053] Put 5g (18mmol) of compound I, 50ml acetone, and 0.5g palladium carbon into the reaction bottle, replace with nitrogen for at least 3 times, and replace with hydrogen for 3 times. Replaced with nitrogen 3 times, filtered with suction, washed the filter cake with acetone, cooled the filtrate to 0°C, added 9.7g (72mmol) of benzyldimethylamine, and added 3.5g (36mmol) of sulfuric acid dropwise. Keep warm for 2 hours, and the reaction is complete. The reaction solution was decompressed to dryness, added 50ml of dichloromethane, 30ml of pure water, separated the phases, extracted the aqueous phase once with 20ml of dichloromethane, combined the organic phases, decompressed to dryness, added 25ml of ethanol, dissolved at 20-35°C, Add sodium isooctanoate ethanol solution dropwise, solids precipitate out, keep warm at 20-35°C for 2 hours, filter with suction, rinse with absolute ethanol, and dry. 4.0 g of compound IV was obtained with a yield of 77% and a content of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com