Avibactam and cefmenoxime compound powder injection for injection and preparation method thereof
A technology of avibactam sodium and cefmenoxime hydrochloride, which is applied in the field of avibactam-cefamenoxime compound powder injection for injection and its preparation, which is easy to use, less resistant to β-lactamase bacteria, and simple in process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
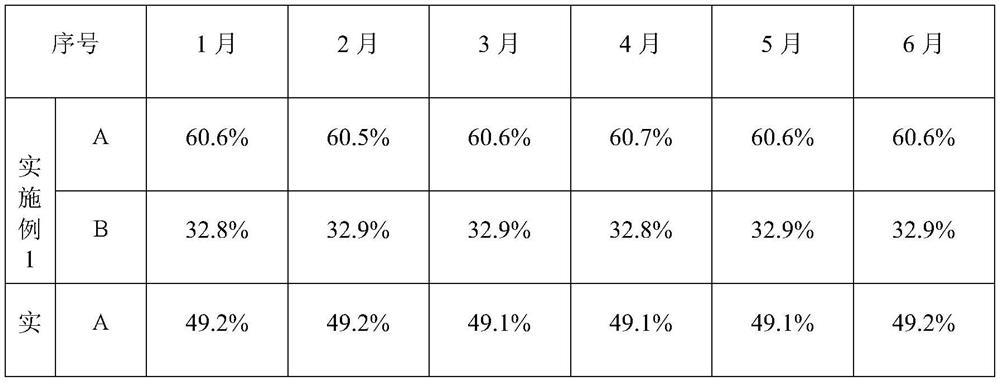
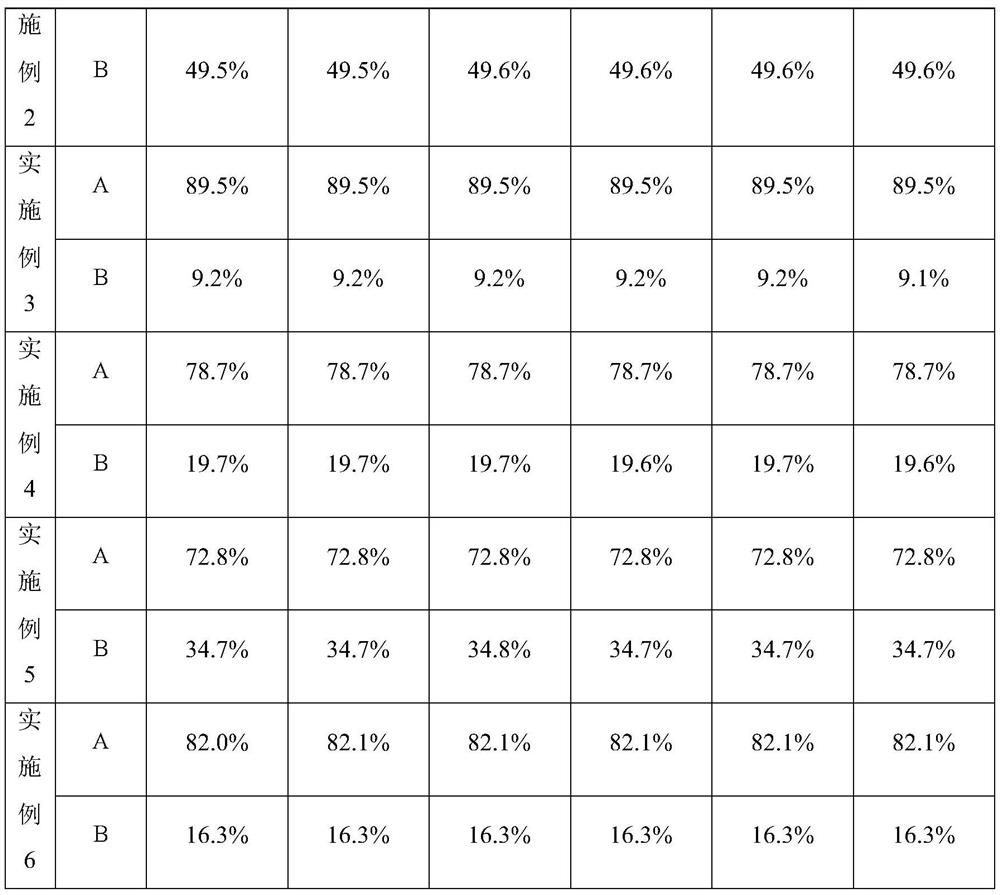
[0017] Under Class B clean environment conditions, 10 kilograms of cefmenoxime hydrochloride powder and 5 kilograms of avibactam sodium powder were mixed and added to a dry granulator with a roll pressure of 10.2 MPa and a roll speed of 95 rpm to be pressed into thin flakes. Grind into granules, then press into thin flakes with a dry granulator under the same conditions, pulverize into granules, and pulverize with a high-speed pulverizer to obtain 13.621 kg of powder (above 60 mesh), which is divided into 13406 bottles (1 gram per bottle). As detected by HPLC, the content of cefmenoxime hydrochloride was 60.6%, and the content of avibactam sodium was 32.9%. The sample was prepared with water for injection, and the dissolution was completed in 1 minute and 38 seconds.
Embodiment 2
[0019] Under Class B clean environment conditions, 10 kilograms of cefmenoxime hydrochloride powder and 10 kilograms of avibactam sodium powder were mixed and added into a dry granulator with a roll pressure of 11.6 MPa and a roll speed of 105 rpm to be pressed into flakes. Pulverize into granules, then press into flakes with a dry granulator under the same conditions, pulverize into granules, and pulverize with a high-speed pulverizer to obtain 18.948 kg of powder (above 60 mesh), which is divided into 18532 bottles (1 gram per bottle). HPLC detection cefmenoxime hydrochloride 49.1%, avibactam sodium 49.6%. The sample was prepared with water for injection, and the dissolution was completed in 1 minute and 03 seconds.
Embodiment 3
[0021] Under Class B clean environment conditions, 10 kg of cefmenoxime hydrochloride powder and 1 kg of avibactam sodium powder were mixed and added to a dry granulator with a roll pressure of 11.7 MPa and a roll speed of 92 rpm to be pressed into flakes. Pulverize into granules, then press into flakes with a dry granulator under the same conditions, pulverize into granules, and pulverize with a high-speed pulverizer to obtain 10.652 kg (above 60 mesh), which is divided into 20893 bottles (0.5 grams per bottle). HPLC detection cefmenoxime hydrochloride 89.5%, avibactam sodium 9.1%. The sample was prepared with water for injection, and the dissolution was completed in 1 minute and 58 seconds.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com