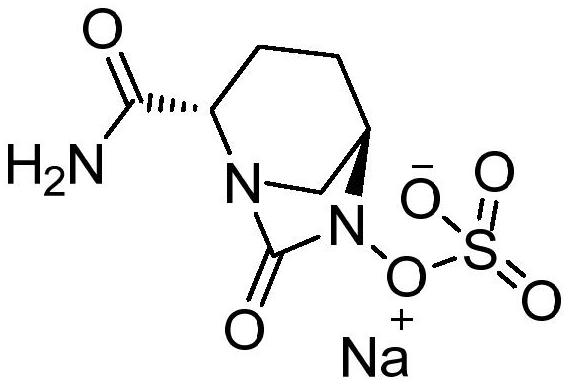
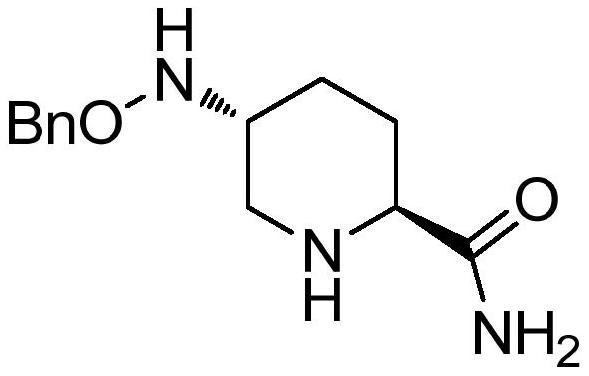
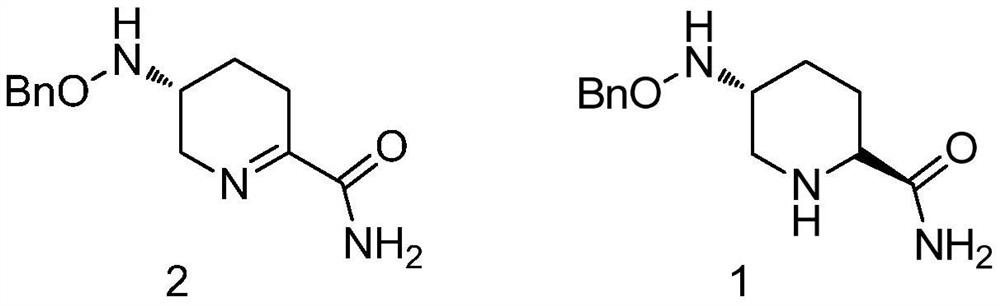
The preparation method of avibactam intermediate
A technology of intermediates and compounds, applied in the field of organic chemistry, can solve the problems of unfriendly environment and low yield, and achieve the effects of environmental friendliness, high purity and high chiral purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] A kind of preparation method of avibactam intermediate, comprises the following steps:
[0034] mixing a compound having a structure shown in formula 2, a chiral catalyst, an acid and a solvent, and stirring to prepare a compound having a structure shown in formula 1;
[0035]
[0036] Described chiral catalyst has the structure shown in formula (I):
[0037]
[0038] The R is selected from a hydrogen atom, a straight-chain alkyl group with 1-20 carbon atoms, a branched-chain alkyl group with 3-20 carbon atoms, a cycloalkyl group with 3-10 carbon atoms, or a substituted or unsubstituted Substituted phenyl.
[0039] The reaction scheme of said method is as follows:
[0040]
[0041] Among them, the chiral catalyst of the present invention is a biomimetic system green catalyst, belonging to the category of organic catalysis, which can avoid transition metal residues caused by the use of transition metals, reduce the pollution of transition metals, especially to...
Embodiment 1
[0073] This embodiment provides a kind of preparation method of avibactam intermediate, the steps are as follows:
[0074] (1) Preparation of chiral catalyst
[0075]
[0076] 2,2,6-Trimethyl-1,3-dioxin-4-one (142.2 mg, 1 mmol) was added dropwise to (S)-α-hydroxy-N-methyl-2-phenylacetamide ( 165.1mg, 1mmol) in a solution of toluene (0.5mL). After stirring at reflux overnight, the reaction mixture was cooled to 50°C and the solvent was removed in vacuo. The crude product was purified by silica gel column chromatography (the volume ratio of n-hexane:ethyl acetate was 20:1) to obtain 187.0 mg of white solid compound a with a yield of 75%.
[0077] The above white solid compound a (498.6mg, 2mmol), ammonium acetate (77.1mg, 1mmol) and hexamethylenetetramine (140.2mg) were dissolved in dioxane (5mL), and heated at 100°C for 30 minutes . The reaction temperature was cooled to normal temperature, water was added, and extracted with dichloromethane. After the organic phase was...
Embodiment 2
[0082] This embodiment provides a preparation method of an avibactam intermediate, which is basically the same as in Example 1, the difference being that the structure of the chiral catalyst is different, and the steps are as follows:
[0083] (1) Preparation of chiral catalyst
[0084]
[0085] 2,2,6-Trimethyl-1,3-dioxin-4-one (142.2 mg, 1 mmol) was added dropwise to (S)-α-hydroxy-N-ethyl-2-phenylacetamide ( 179.2mg, 1mmol) in a solution of toluene (0.5mL). After stirring at reflux overnight, the reaction mixture was cooled to 50°C and the solvent was removed in vacuo. The crude product was purified by silica gel column chromatography (the volume ratio of n-hexane:ethyl acetate was 20:1) to obtain 243.0 mg of white solid compound b with a yield of 82%.
[0086] The above white solid compound b (526.6mg, 2mmol), ammonium acetate (77.1mg, 1mmol) and hexamethylenetetramine (140.2mg) were dissolved in dioxane (5mL), and heated at 100°C for 30 minutes . The reaction tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com