Preparation method of avibactam intermediate
A technology for intermediates and compounds, applied in the field of pharmaceutical synthesis, can solve the problems of weak carbonate alkalinity, high preparation cost, long reaction time, etc., and achieves the effects of mild reaction conditions, avoiding potential safety hazards and fewer reaction impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
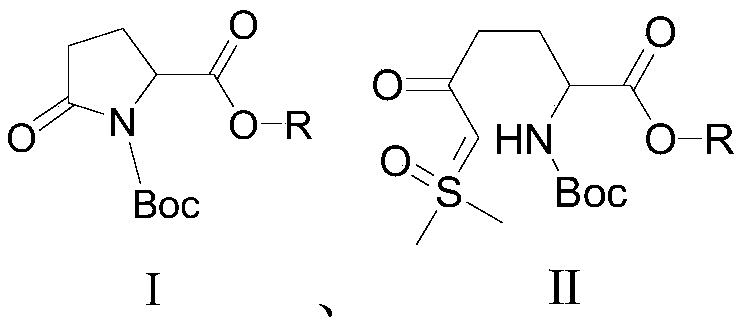
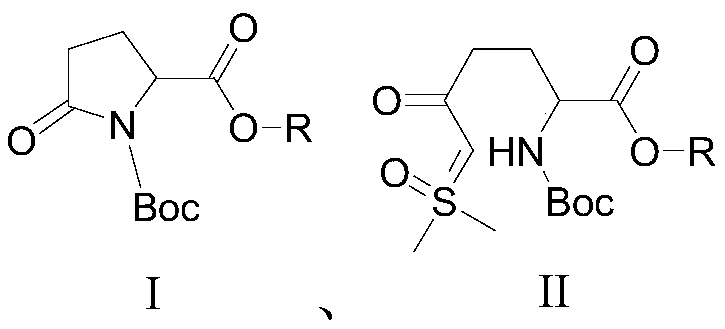
[0021] A preparation method of an avibactam intermediate, compound I and trimethylsulfoxide iodide are reacted in a solvent in the presence of a base to prepare compound II, and the reaction is as follows:
[0022]
[0023] The operation is as follows:
[0024] Put 25g (78mmol) of Compound I, 100ml of dimethyl sulfoxide, 21.1g (96mmol) of trimethylsulfoxide iodide, 2.5g (104mmol) of lithium hydroxide into the reaction bottle, and keep warm at 30°C until the reaction is complete (about 2 hours ), add 20ml of pure water, 60ml of saturated ammonium chloride, 200ml of EA, separate the phases, extract the aqueous phase once with 50ml of EA, combine the organic phases, wash twice with saturated brine, reduce the pressure to dryness, crystallize with isopropyl ether, pump Filter and dry to obtain 30.9g of compound II, yield: 96%, content: 97%.
Embodiment 2
[0026] A preparation method of an avibactam intermediate, compound I and trimethylsulfoxide iodide are reacted in a solvent in the presence of a base to prepare compound II, and the reaction is as follows:
[0027]
[0028] The operation is as follows:
[0029] Put 20.4g (78mmol) of Compound I, 100ml of dimethyl sulfoxide, 21.1g (96mmol) of trimethylsulfoxide iodide, 2.5g (104mmol) of lithium hydroxide into the reaction bottle, and keep warm at 30°C until the reaction is complete (about 1.5 hours), add 20ml of pure water, 60ml of saturated ammonium chloride, 100ml of EA, separate the phases, extract the aqueous phase once with 50ml of EA, combine the organic phases, wash twice with saturated brine, reduce the pressure to dryness, and crystallize with isopropyl ether, Suction filtration and drying gave Compound II 26.1g, yield: 94%, content: 97%.
Embodiment 3
[0031] A preparation method of an avibactam intermediate, compound I and trimethylsulfoxide iodide are reacted in a solvent in the presence of a base to prepare compound II, and the reaction is as follows:
[0032]
[0033] The operation is as follows:
[0034] Put 100ml of dimethylsulfoxide, 21.1g (96mmol) of trimethylsulfoxide iodide, 25g (78mmol) of compound I, 5.0g (85.8mmol) of magnesium hydroxide into the reaction bottle, and keep warm at 30°C until the reaction is complete (about 2.5 hours), add 20ml of pure water, 60ml of saturated ammonium chloride, and 100ml of EA, separate the phases, extract the water phase once with 50ml of EA, combine the organic phases, wash twice with saturated brine, depressurize to dryness, crystallize with isopropyl ether, pump Filter and dry to obtain 31.2g of compound II, content: 96%, yield: 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com