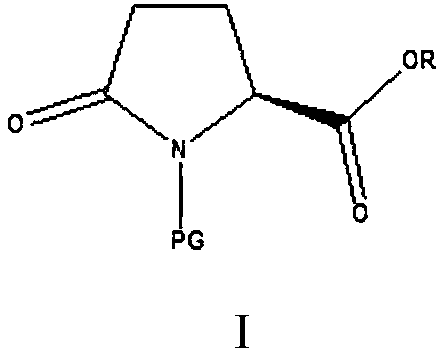
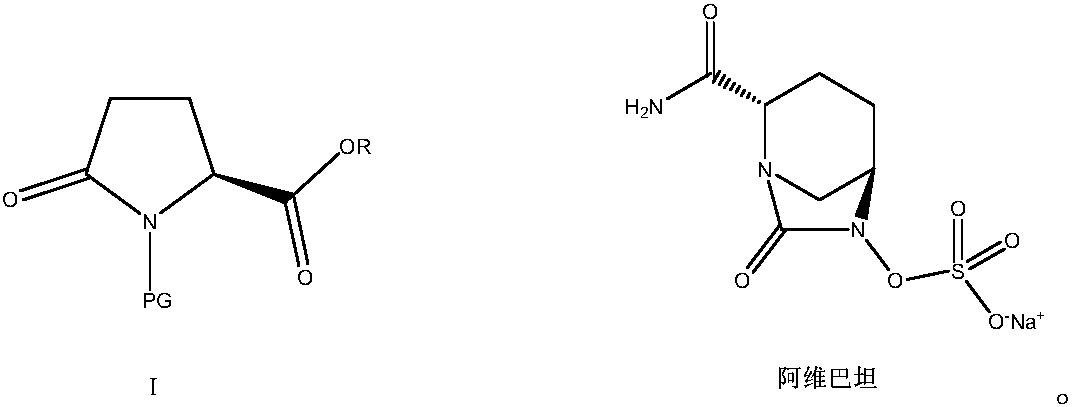
Green preparation method of N-substituted-L-pyroglutamate
A technology of pyroglutamate and glutamate diester hydrochloride, which is applied in the field of green preparation of N-substituted-L-pyroglutamate, can solve unfavorable green production, cumbersome post-processing, and long process routes and other problems, to achieve the effect of classic reaction type, short process route and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
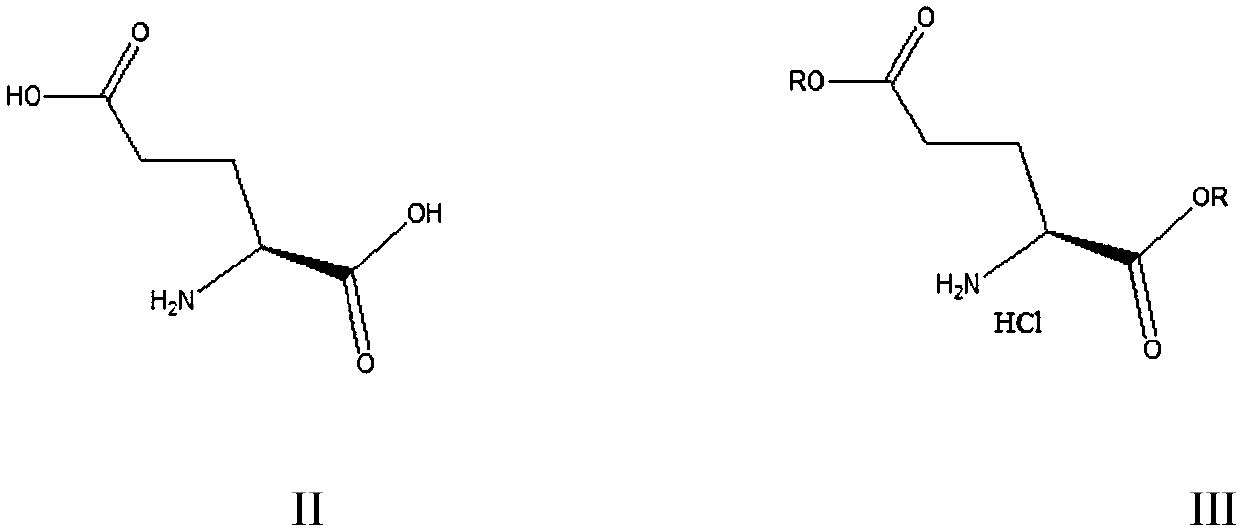
[0047] Embodiment 1: L-glutamic acid dibenzyl ester hydrochloride (Ⅲ 1 ) preparation
[0048] Add 280 grams of benzyl alcohol, 14.7 grams (0.10 moles) of L-glutamic acid, 30.0 grams (0.25 mol) thionyl chloride, heated, reacted at 80-85°C for 5 hours, cooled to 20-25°C, replaced hydrogen chloride gas in the system with nitrogen, and after 30 minutes of replacement, recovered excess thionyl chloride and benzyl alcohol by distillation, and then Add 120 g of methyl tert-butyl ether to the residue, beat, filter, and dry to obtain 35.9 g of white solid L-dibenzyl glutamate hydrochloride with a liquid phase purity of 99.8% and a yield of 98.7%.
Embodiment 2
[0049] Embodiment 2: L-glutamic acid dibenzyl ester hydrochloride (Ⅲ 1 ) preparation
[0050]Add 280 grams of benzyl alcohol, 14.7 grams (0.10 moles) of L-glutamic acid, 30.0 grams (0.15 mol) diphosgene, heated, reacted at 70-75°C for 6 hours, cooled to 20-25°C, and replaced the hydrogen chloride gas in the system with nitrogen for 30 minutes. Recover excessive diphosgene and benzyl alcohol by distillation, then add 120 grams of methyl tert-butyl ether to the residue, make a slurry, filter, and dry to obtain 35.5 grams of white solid L-dibenzyl glutamate hydrochloride, liquid phase The purity is 99.7%, and the yield is 97.6%.
Embodiment 3
[0051] Embodiment 3: L-glutamic acid diethyl ester hydrochloride (Ⅲ 2 ) preparation
[0052] Add 300 grams of ethanol, 14.7 grams (0.10 moles) of L-glutamic acid, 25.0 grams (0.08 moles) ) Triphosgene, heating, reacting at 70-75° C. for 5 hours, cooling to 20-25° C., replacing hydrogen chloride gas in the system with nitrogen, and replacing it for 30 minutes. Recover excess triphosgene and ethanol by distillation, then add 100 grams of methyl tert-butyl ether to the residue, make a slurry, filter, and dry to obtain 23.5 grams of white solid L-diethyl glutamate hydrochloride with a liquid phase purity of 99.7 %, the yield is 98.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com