Synthesis method of avibactam intermediate (I)
A synthesis method and a technology for intermediates, which are applied in the field of synthesis of avibactam intermediates, can solve the problems of expensive raw materials, complicated synthesis steps and the like, and achieve simple operation, high chemical yield and optical purity, and good enantioselectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] A kind of synthetic method of avibactam intermediate (I), comprises steps:
[0034] Dissolving the rhodium metal catalyst and the chiral bisphosphine ligand in a solvent to obtain a mixed solution, adding compound (II) and hydrogen gas to the mixed solution for an asymmetric hydrogenation reaction to prepare the avibactam intermediate (I);
[0035] The synthetic route of Avibactam intermediate (I) is as follows:
[0036]
[0037] in:
[0038] Compound (II) is 6-bromo-2-((tert-butoxycarbonyl)amino)-5,5-dimethoxyhex-2-enoic acid methyl ester, and the chemical structural formula of compound (II) is In the formula, X is halogen, R2 is any one of C1-C5 alkyl, aralkyl, and aryl, and R3 is various alkoxycarbonyl groups;
[0039] Avibactam intermediate (I) is (S)-6-bromo-2-((tert-butoxycarbonyl)amino)-5,5-dimethoxyhexanoic acid methyl ester, Avibactam intermediate The chemical structural formula of (I) is
[0040] In this method, the enamine substrate, compound (II), ...
Embodiment 1
[0062] Preparation of Compound (Ia)
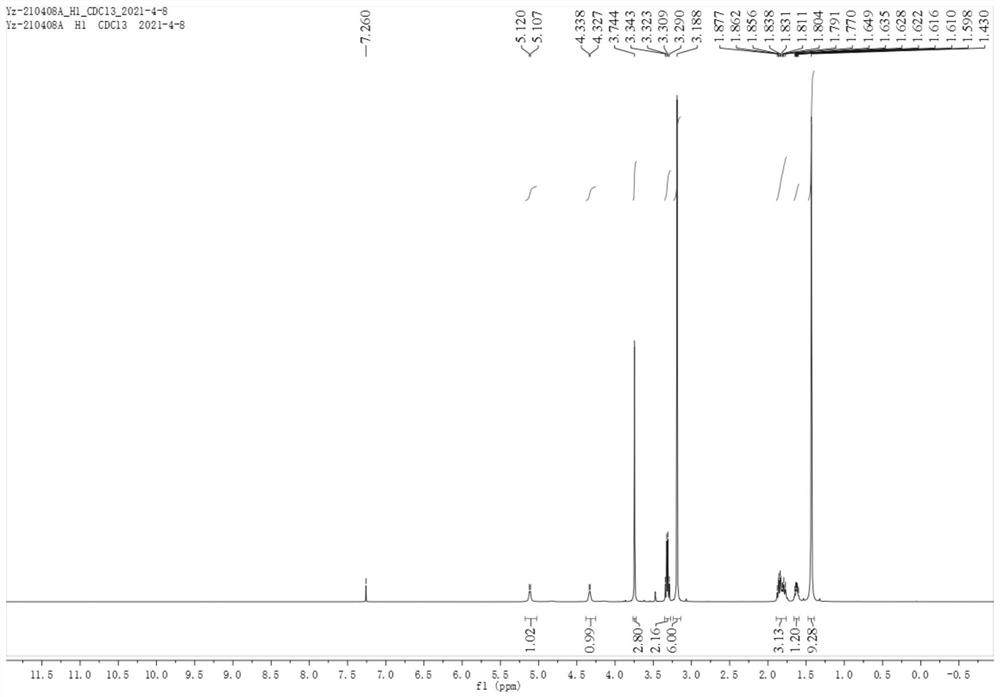
[0063] Dissolve 106 mg of bis(1,5-cyclooctadiene) rhodium tetrafluoroborate, 617 mg of ligand (R)-(-)-DTBM-SegPhos and 15 mL of degassed methanol in a dry reaction flask, stir for 30 min, and then Add 1g of 6-bromo-2-((tert-butoxycarbonyl)amino)-5,5-dimethoxyhex-2-enoic acid methyl ester in the reaction flask, place it in the autoclave, replace with hydrogen for 3 Once, hydrogen gas was introduced to 40atm, and stirred at 25°C for 48h. Hydrogen was released slowly, the solvent was removed under reduced pressure, and the white solid obtained by separation with a silica gel column was the avibactam intermediate (Ia) (83% yield, 98% ee).
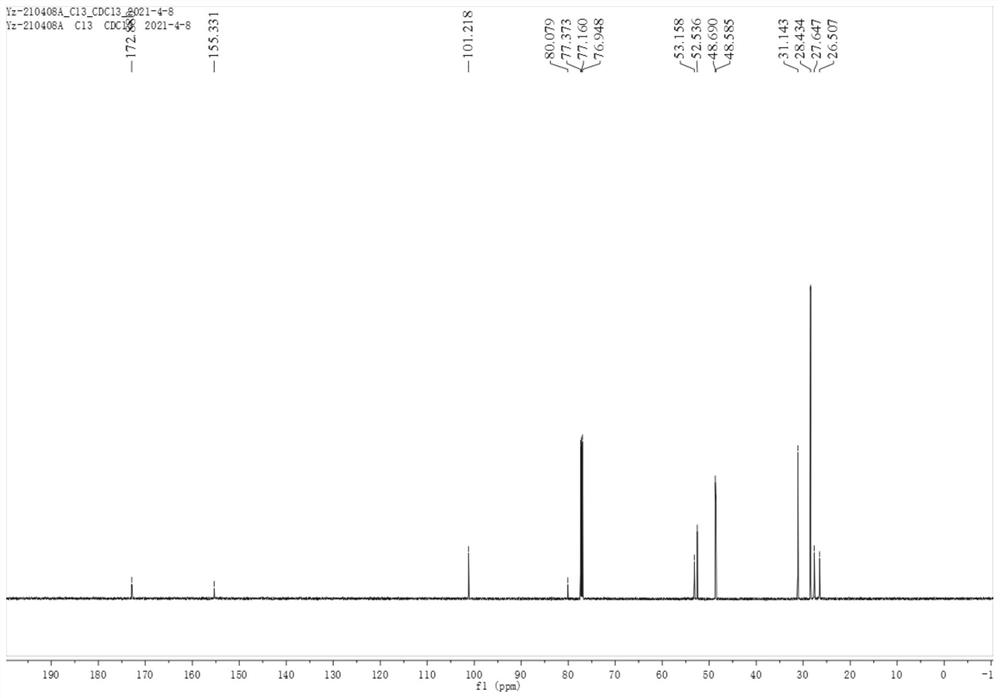
[0064] figure 1 is compound (Ia) hydrogen spectrogram; figure 2 It is the carbon spectrum of compound (Ia).
[0065] 1 H NMR (400MHz, Chloroform-d) δ5.11(d, J=5.2Hz, 1H), 4.33(m, 1H), 3.74(s, 3H), 3.35–3.27(m, 2H), 3.19(s, 6H), 1.89–1.76(m,3H), 1.66–1.59(m,1H), 1.43(s,9H).
[0066] 13 C NMR (100MHz...
Embodiment 2
[0068] Preparation of compound (Ib)
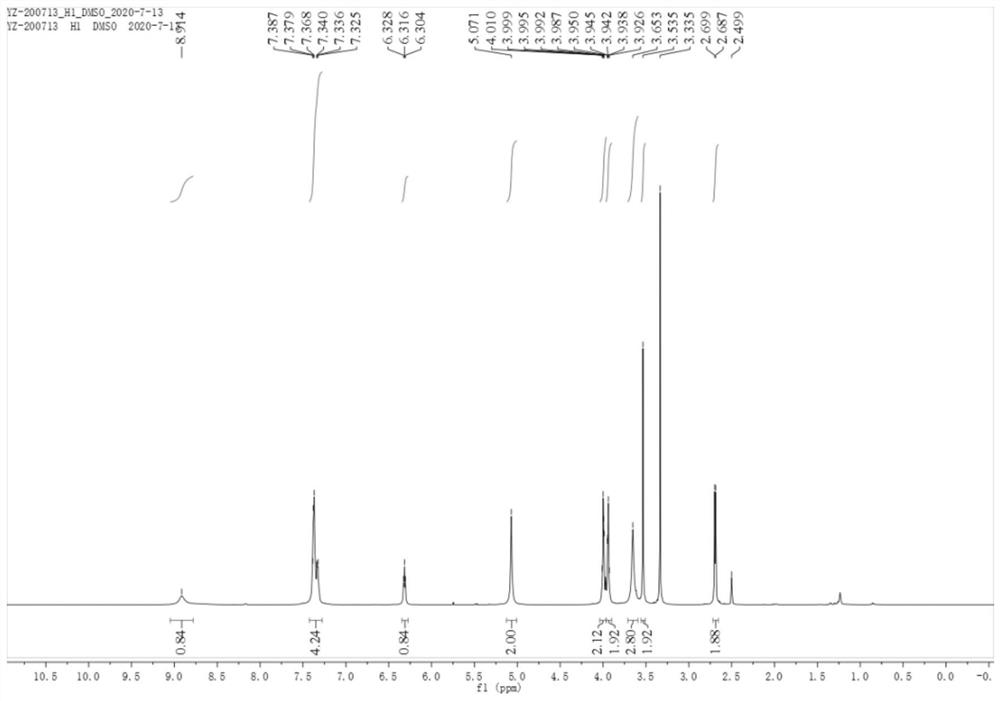
[0069] Dissolve 20mg of bis(1,5-cyclooctadiene)rhodium tetrafluoroborate, 29mg of ligand (R,R)-QuinoxP* and 6mL of degassed methanol in a dry reaction flask, stir for 30min, and then pour into the reaction flask Add 200 mg of (S)-4-(2-(bromomethyl)-1,3-dioxolan-2-yl)-2-((tert-butoxy) and stir at room temperature for 48 hours at 25°C. Release slowly Hydrogen, the solvent was removed under reduced pressure, and the white solid obtained by recrystallization was the avibactam intermediate (Ib) (87% yield, 98% ee).
[0070] image 3 is compound (Ib) hydrogen spectrogram; Figure 4 It is the carbon spectrum of compound (Ib).
[0071] 1 H NMR (600MHz, DMSO-d6) δ8.91(s, 1H), 7.43–7.28(m, 5H), 6.32(t, J=7.2Hz, 1H), 5.07(s, 2H), 4.04–3.96( m,2H),3.96–3.90(m,2H),3.65(s,3H),3.53(s,2H),2.69(d,J=7.2Hz,2H).
[0072] 13 C NMR (151MHz, DMSO-d6) δ164.59, 154.23, 136.62, 129.28, 129.13, 128.37, 127.92, 127.73, 107.35, 65.90, 65.32, 52.03, 36.05, 33.83...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com