Preparation method of avibactam sodium
A crystallization method and compound technology, applied in the field of preparation of Avibatana, can solve the problems of low yield, long reaction route, unsuitable for industrialization and the like, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
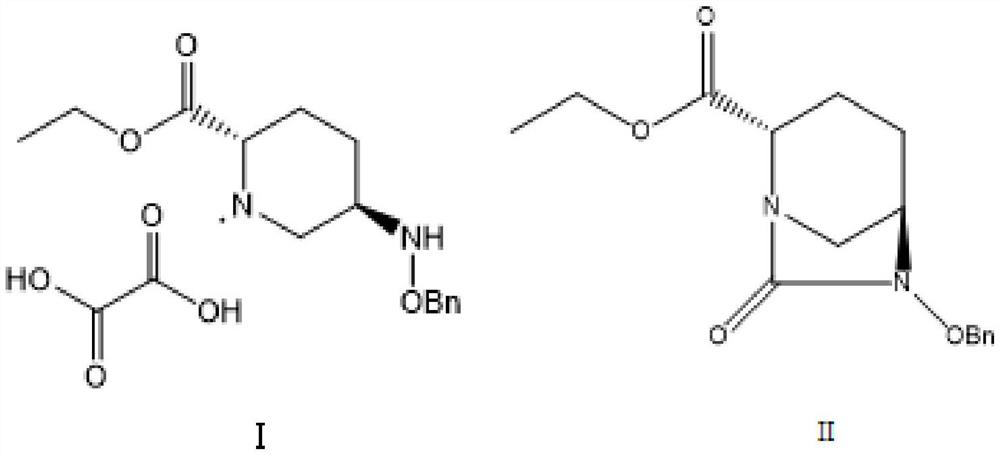
[0038] The synthesis of embodiment 1 intermediate II
[0039] Add 1000g of starting material I, 8L of water, and 6L of dichloromethane to a 20L reactor in sequence, stir to dissolve, add 548.6g of NaHCO3 in batches, stir at room temperature for 1 hour, let stand to separate layers, take the dichloromethane layer, and wash the water layer with dichloromethane Extracted with methane, combined dichloromethane layers, dried with saturated brine and anhydrous sodium sulfate successively, distilled dichloromethane under reduced pressure, added 3L ethyl acetate, 421.4g DIPEA (N,N-diisopropylethylamine), controlled Warm at 10°C-20°C, slowly add 690.7g of Fmoc-Cl 3L ethyl acetate solution dropwise to the reaction liquid, react at room temperature for 1h after dropping, add 162.15g CDI to the reaction liquid, stir and react at 40-50°C for 14h, Add 497.4g of diethylamine to the reaction liquid and react at room temperature for 10h, filter, wash the filtrate with hydrochloric acid and sat...
Embodiment 2
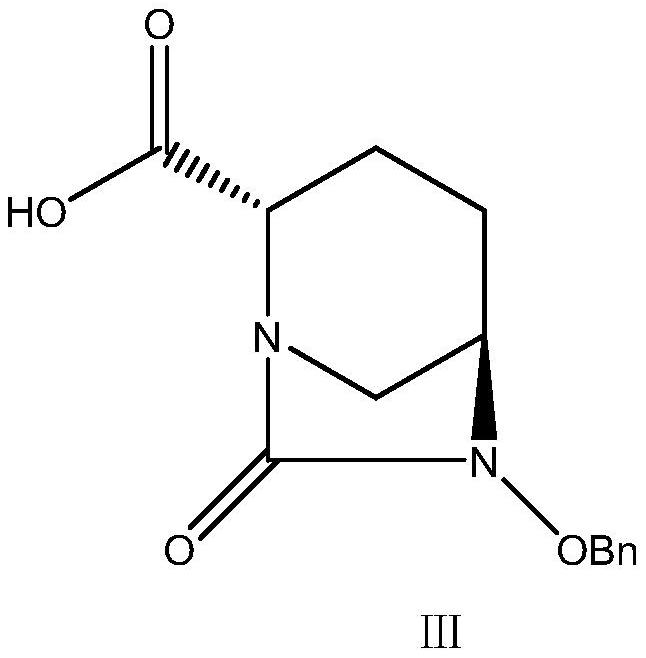
[0040] Embodiment 2, the synthesis of intermediate III
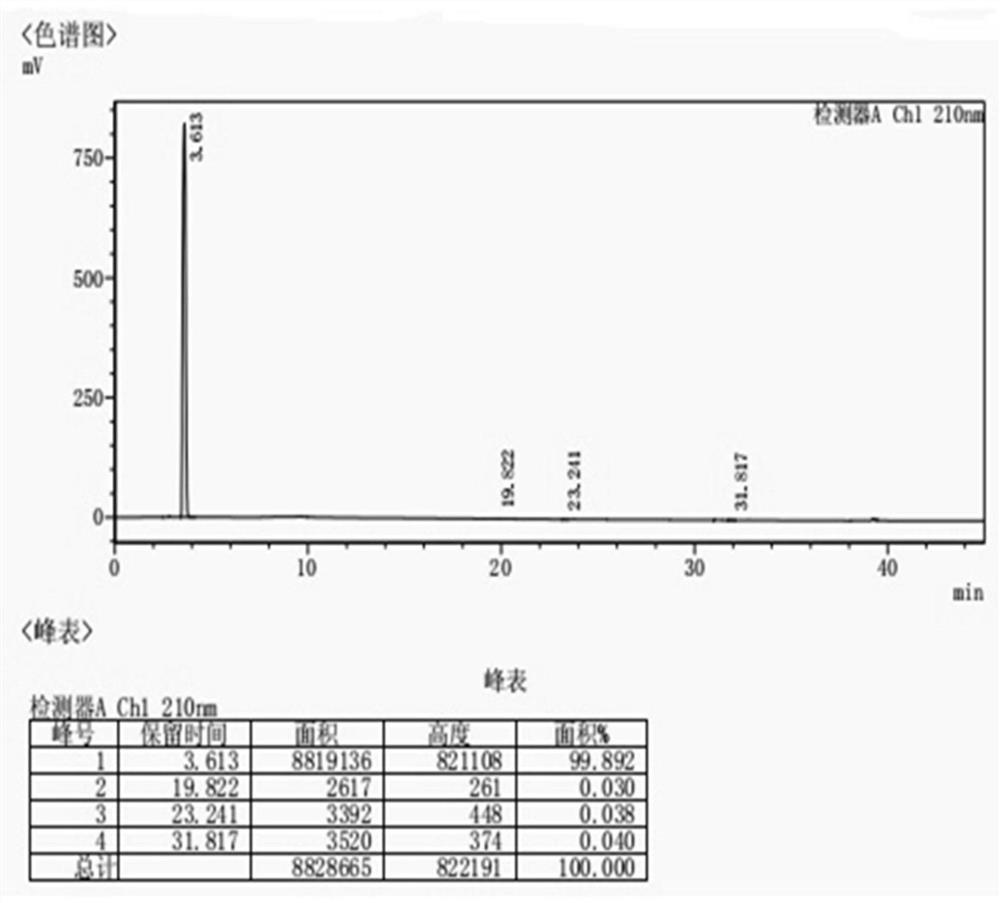
[0041] Add 2.75L tetrahydrofuran, 1.1L tap water, and 550g intermediate II to a 20L reactor, stir to dissolve, cool down to -5-0°C, control the temperature at 0-5°C, and add dropwise 1.65L aqueous solution of 79.6g lithium hydroxide monohydrate, Insulate and react for 1 hour, add 2.75L ethyl acetate, let stand to separate and take the lower water phase, extract the water phase with ethyl acetate, take the water phase, adjust the pH value to 2-3 with 1N hydrochloric acid in the water phase, and extract with ethyl acetate , take the ethyl acetate layer, dry it with saturated brine and anhydrous sodium sulfate successively, filter, and distill the filtrate under reduced pressure at 35-40°C, add 11.0L of n-heptane dropwise, crystallize at -5±5°C for 1h, filter, 40 °C drying to obtain an off-white solid with a yield of 60-70%.
Embodiment 3
[0042] Embodiment 3, the synthesis of intermediate IV
[0043] Add 6L of dichloromethane and 300g of intermediate III to a 20L reactor, stir to dissolve, add 164.8g of triethylamine, cool down to below -10°C, add 222.5g of isobutyl chloroformate dropwise below -5°C, dropwise add After the completion, continue to lower the temperature to below -10°C, add 0.9L ammonia water dropwise below -10°C, stir at room temperature for 1 hour after the dropwise addition is completed, add 3.0L drinking water, let stand to separate and take the organic layer, and the organic layer is sequentially washed with 1N hydrochloric acid, Wash with saturated brine, take the organic layer, dry with anhydrous sodium sulfate, filter, distill the filtrate under reduced pressure at 35-40°C, add 6L of n-heptane dropwise, stir and crystallize at 20-25°C for 2h, filter and dry to obtain an off-white solid, and collect The rate is 75-85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com