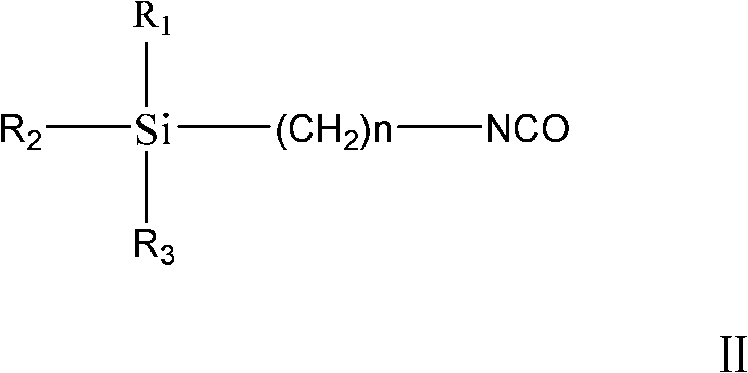
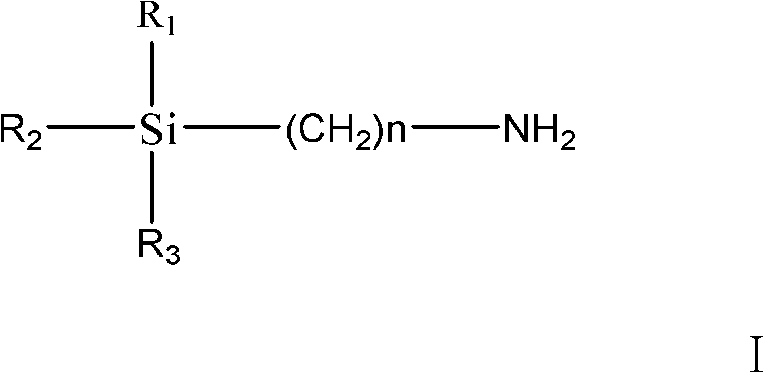Method of preparing trialkoxysilanes isocyanic ester
A technology of trialkoxysilyl isocyanate and trialkoxysilyl propyl isocyanate, which is applied in the field of preparation of trialkoxysilyl isocyanate, can solve the problems of unsafe and reliable, weak operability, etc., and avoid Effect of phosgene, low price, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The present embodiment is the preparation method of triethoxysilyl propyl isocyanate, specifically comprises the following steps:
[0022] ① Put a 2L reaction device with a stirrer and a thermometer into an ice-salt bath, and control the temperature 3 Dissolve in 600ml of water, then add to the reaction device and stir, and control the temperature at 5°C to 0°C.
[0023] ②Dissolve 25.4g of triphosgene in 400ml of CH 2 Cl 2 in, and then added to the reaction device; 45.12 g of triethoxysilylpropylamine was dissolved in 400 ml of CH 2 Cl 2 Then add it to the reaction device, stir and react for 15 minutes, and control the temperature at -5°C to 10°C.
[0024] ③ Place the reacted solution to separate the phases into aqueous phase and organic phase, and use 100ml of CH for the aqueous phase 2 Cl 2 Extract twice, combine the organic phase obtained after extraction and the organic phase obtained during phase separation, then dry, filter with suction, and remove CH 2 Cl ...
Embodiment 2~ Embodiment 5
[0027] The preparation method of each embodiment is basically the same as that of Example 1, and the specific parameters of each embodiment are shown in Table 1, wherein the finished product obtained in Example 4 is trimethoxysilylpropyl isocyanate, and all the other are triethoxysilylpropyl isocyanate .
[0028] Example 1
Example 2
Example 3
Example 4
Example 5
Reactor volume
2L
5L
20L
2L
500L
60g
NaHCO 3
160g
NaHCO 3
700g
NaHCO 3
43.1g
NaHCO 3
14kg
NaHCO 3
Water in which chlorine scavenger is dissolved
600ml
2L
7L
600ml
140L
25.4g
60g
250g
25.4g
5kg
dissolved triphosgene
CH 2 Cl 2
400ml
1000ml
3L
400ml
60L
Trialkoxysilylamine
45.12g
Triethoxy
silyl propyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com