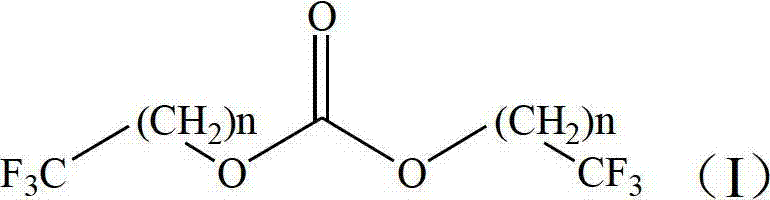
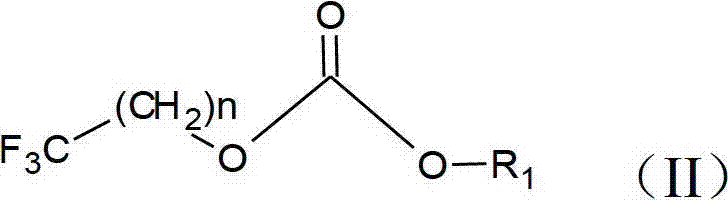
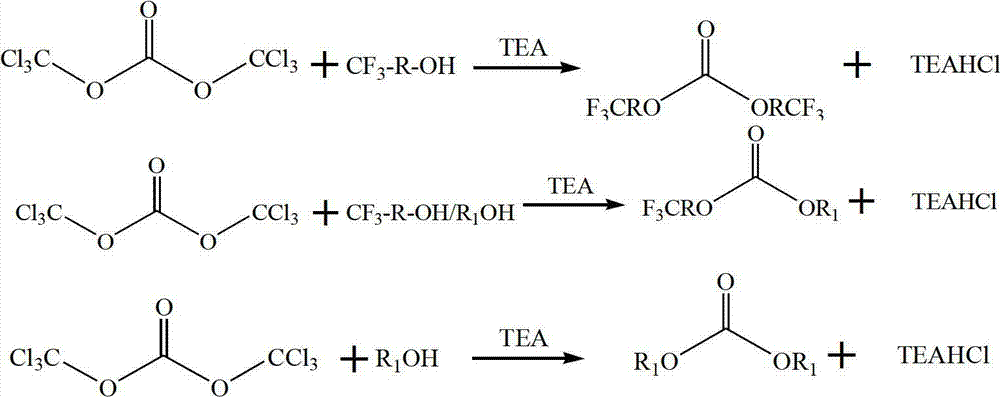
Preparation method of trifluoromethyl straight-chain carbonate
A technology of straight-chain carbonate and trifluoromethyl, applied in the field of preparation of trifluoromethyl-containing straight-chain carbonate, can solve the problem of reduced hydroxyl activity, impact on synthesis yield, and difficulty in separating alcohols, carbonates and transesterification products and other problems, to achieve the effect of strong stability, simple process, cycle performance and high voltage characteristics improvement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 297.0g (1.0mol) of triphosgene and 600.0g (6.0mol) of trifluoroethanol into a three-necked flask, stir and mix well, add 620.0g ((6.14mol) of triethylamine dropwise at 25°C, and keep warm for 4 hours after the addition Finally, the content of trifluoroethanol in the sampling filtrate is less than 1%; cooling and filtration obtains 578.6g of filtrate, through Agilent 7890A, analysis and detection product bistrifluoroethyl carbonate content 93.6178%, triethylamine content 2.8156%, trifluoroethanol 0.4679% The obtained filtrate was rectified under normal pressure with reflux adjustment to recover residual trifluoroethanol and excess trifluoroethanol, collected 523.6 g of bis-trifluoroethyl carbonate at 112~116°C / 100kPa, and analyzed and detected by Agilent 7890A (RT2 .525), the content is 99.94Wt.%, and the yield is 77.2%.
Embodiment 2
[0030] Add 297.0g (1.0mol) of triphosgene and 1368.0g (12.0mol) of trifluoropropanol into a three-necked flask, stir and mix well, add 620.0g (6.14mol) of triethylamine dropwise at 80°C, and keep warm for 10 hours after the addition Finally, triethylamine content is less than 3% in the sampling filtrate; Cooling filtration obtains 1485.6 filtrate, by Agilent 7890A, analysis and detection product bistrifluoropropyl carbonate content 58.6358%, triethylamine content 1.2313%, trifluoropropanol 40.1637% . The obtained filtrate was rectified under normal pressure with reflux regulation to recover residual trifluoropropanol and excess triethylamine, and 815.6 g of bis-trifluoropropyl carbonate at 75~85°C / 10~30kPa was collected under reduced pressure, and analyzed by Agilent 7890A Detection (RT5.596), the content is 99.91Wt.%, and the yield is 95.1%.
Embodiment 3
[0032] Add 297.0g (1.0mol) of triphosgene, 400.0g (4.0mol) of trifluoroethanol and 96.0g of methanol (3.0mol) into a three-necked flask and stir well, then add 452.6g (6.2mol) of n-butylamine dropwise at 50°C, After incubation for 1 hour after the dropwise addition, the content of n-butylamine in the sampled filtrate was less than 3%; 565.8g of filtrate was obtained by cooling and filtration, which was analyzed and detected by Agilent 7890A. The content of methyl trifluoroethyl carbonate was 72.0718%, and the content of n-butylamine was 1.9156 %, trifluoroethanol 18.4679%, bistrifluoroethyl carbonate content 4.1135%, dimethyl carbonate 3.4312%. The obtained filtrate was subjected to rectification under normal pressure with reflux adjustment to recover residual trifluoroethanol and excess n-butylamine, collected 385.4 g of trifluoroethyl methyl carbonate at 102~106°C / 100kPa, and analyzed and detected by Agilent 7890A (RT1. 586), the content is 99.90Wt.%, and the yield is 67.5%....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com