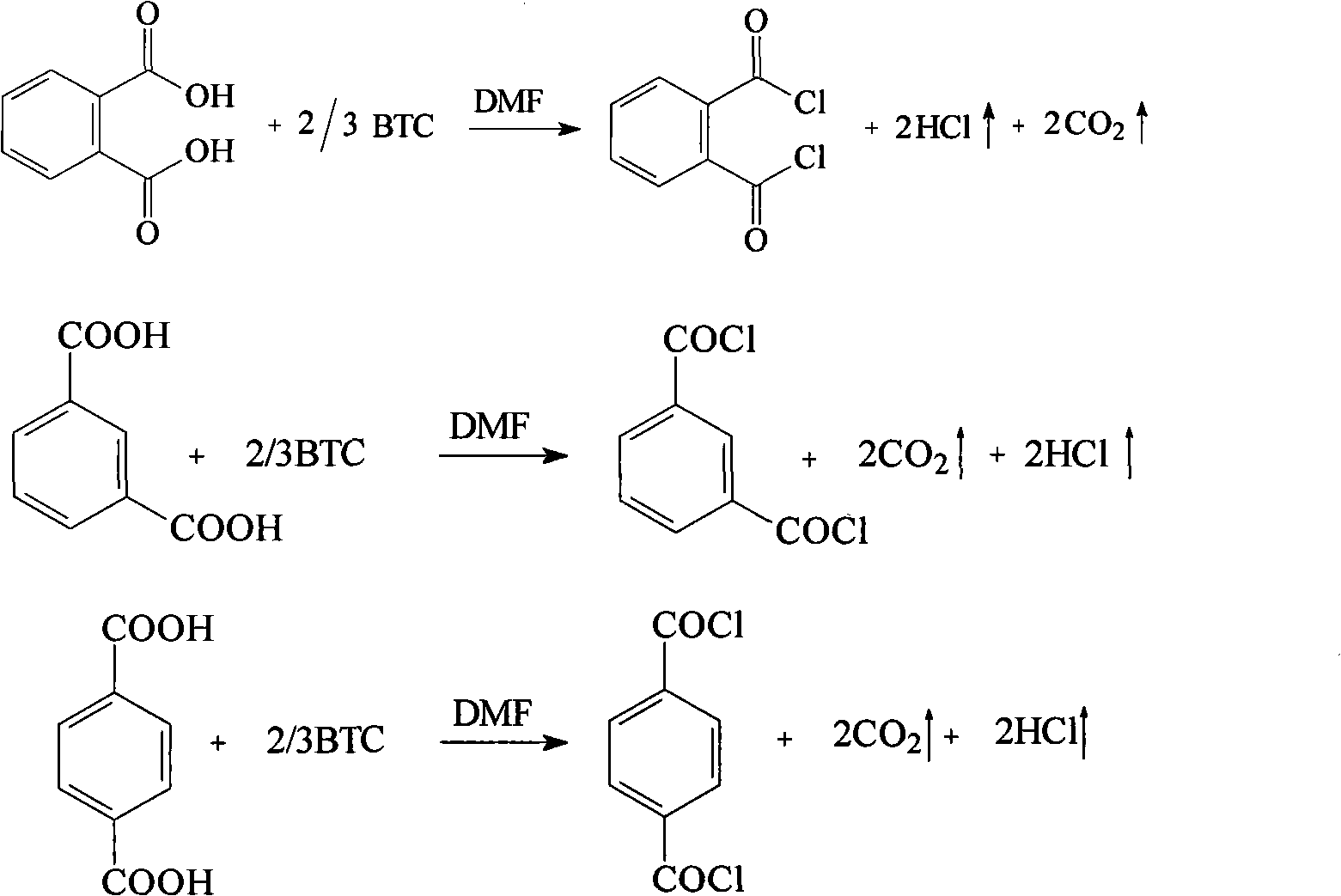
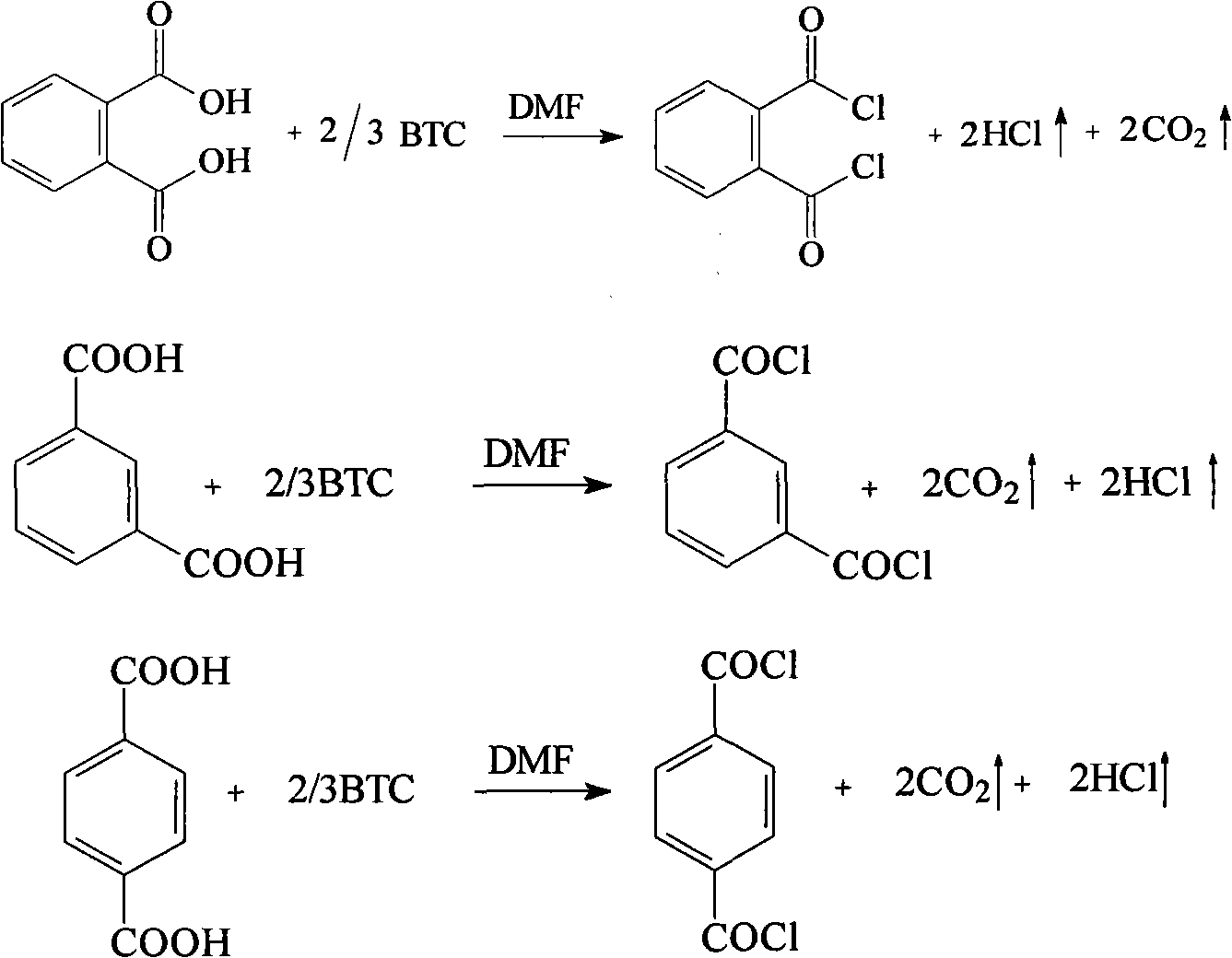
Preparation method for phthalyl chloride, m-phthaloyl chloride and paraphthaloyl chloride
A technology of terephthaloyl chloride and phthalic acid, applied in the preparation of organic compounds, preparation of carboxylate, chemical instruments and methods, etc., can solve the problems of complex post-processing, complex process operation, high temperature time, etc., and achieve Easy industrial production, reasonable process conditions, simple and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0011] Next, solid phosgene is used as chlorinating agent, and the method for preparing o-, m-, and terephthaloyl dichlorides by reacting solid phosgene with o-, m-, and terephthalic acid is further described in detail through specific examples.
[0012] 1. The synthetic embodiment of phthaloyl chloride
[0013] (1) Weigh 8.3g of phthalic acid and put it in a three-necked flask, then add 50mL of solvent 1,2-dichloroethane and 2mL of DMF catalyst, add the solution dissolved in 150mL of 1,2 - 29.7g solid phosgene solution of dichloroethane, after adding, reflux reaction under stirring for 3 hours, filter the reaction solution and collect the 180~195°C / 0.09Mpa fraction by vacuum distillation, which is the product phthaloyl chloride, Yield 70.3%.
[0014] (2) Weigh 8.3g of phthalic acid and place it in a three-necked flask, then add 50mL of solvent 1,2-dichloroethane and 3mL of DMF catalyst, and add 130mL of phthalic acid dissolved in 130mL of 1,2-dichloro 24.7g solid phosgene s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com