Process for synthesizing carbonochloridic acid 9-fluorene methyl ester
A technology of chloroformic acid and a synthetic method, which is applied in the preparation of phosgene or haloformate, organic chemistry, etc., can solve the problems of high price and toxicity, and achieves the effects of mild conditions, simple process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
Embodiment 1
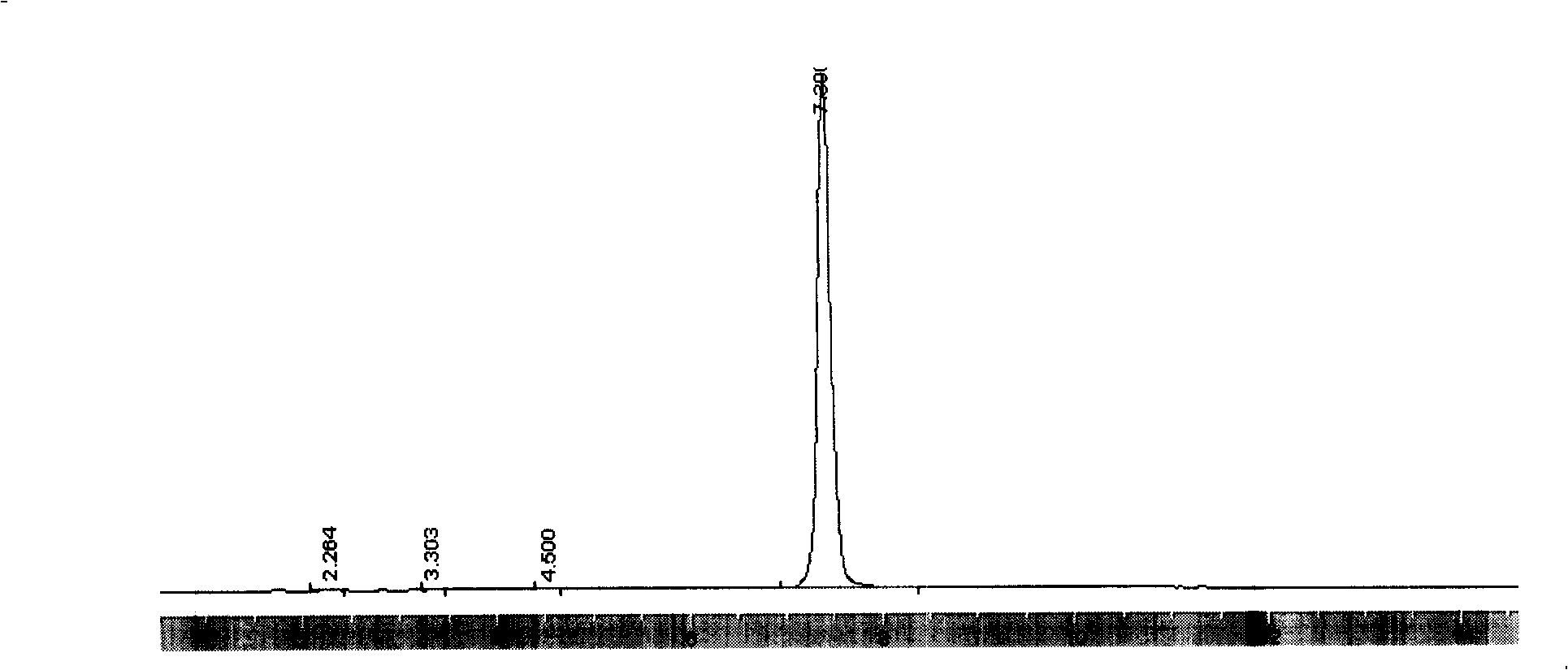
[0026] Embodiment 1: in 250ml there-necked flask, add 7.0 gram solid phosgene, 10 gram purity is the mixed solution (mass ratio is 5: 95) of 99% 9-fluorenemethanol and 100ml toluene and normal hexane, stir in-5 ℃ , add 1.7 g of diethylamine, react for 2 hours, then heat up to 25-30°C, stir for 2 hours, filter to remove solids. The obtained filtrate was cooled in an ice bath for 3 hours, after Fmoc-Cl was fully crystallized and separated out, filtered and dried to obtain 10.52 grams of 9-fluorenylmethyl chloroformate product with a purity of 98.12% (the chromatogram is shown in figure 1 ), yield 79.21%.
Embodiment 2
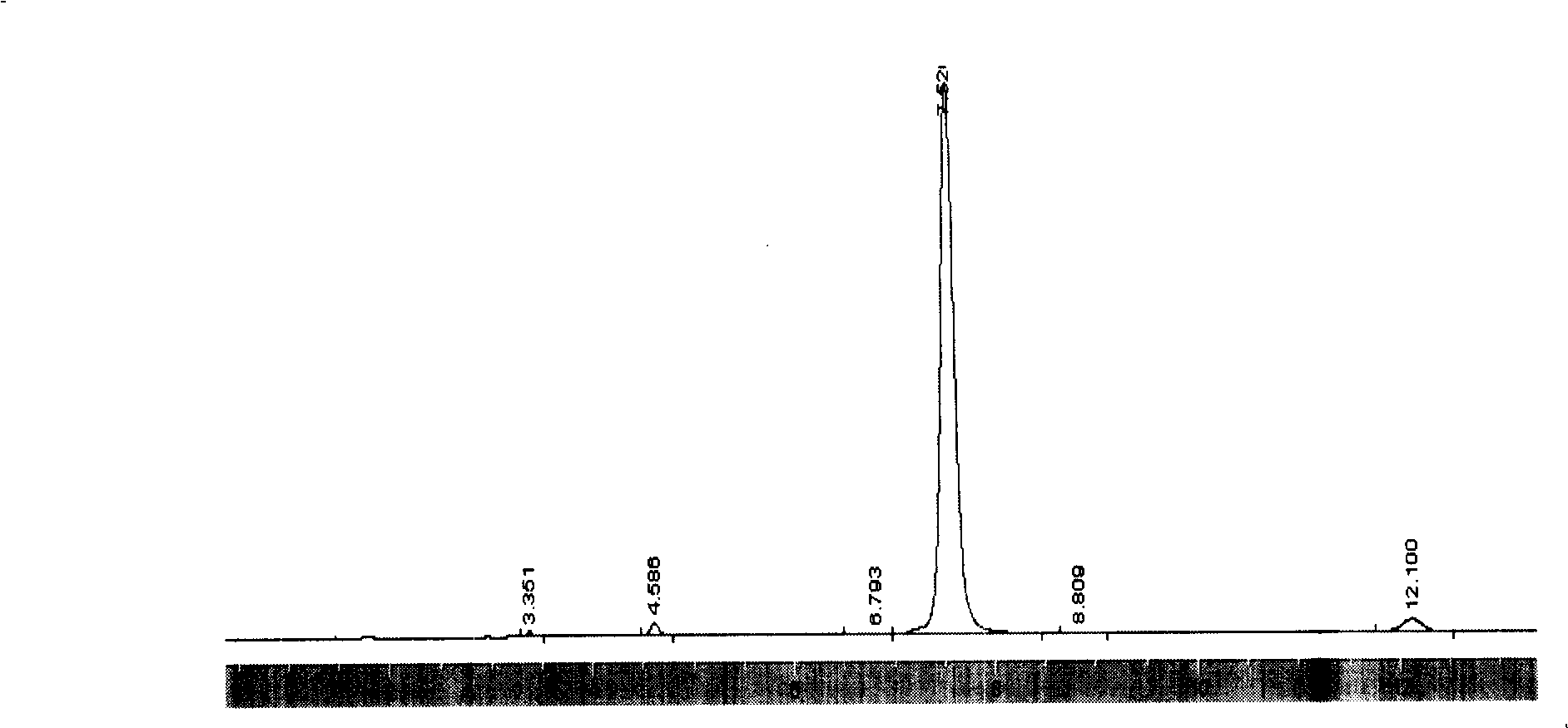
[0027] Embodiment 2: in 250ml there-necked flask, add 7.0 gram solid phosgene, 10 gram purity be the mixed solution (mass ratio is 5: 95, gas chromatography detection, prepared), stirred at -5°C, added 1.7 g of diethylamine, reacted for 2 hours, raised the temperature to 25-30°C, stirred for 2 hours, and filtered to remove solids. The obtained filtrate was cooled in an ice bath for 3 hours, after Fmoc-Cl was fully crystallized and separated out, filtered and dried to obtain 10.40 grams of 9-fluorenylmethyl chloroformate product with a purity of 98.05% (the chromatogram is shown in figure 2 ), yield 78.25%.
Embodiment 3
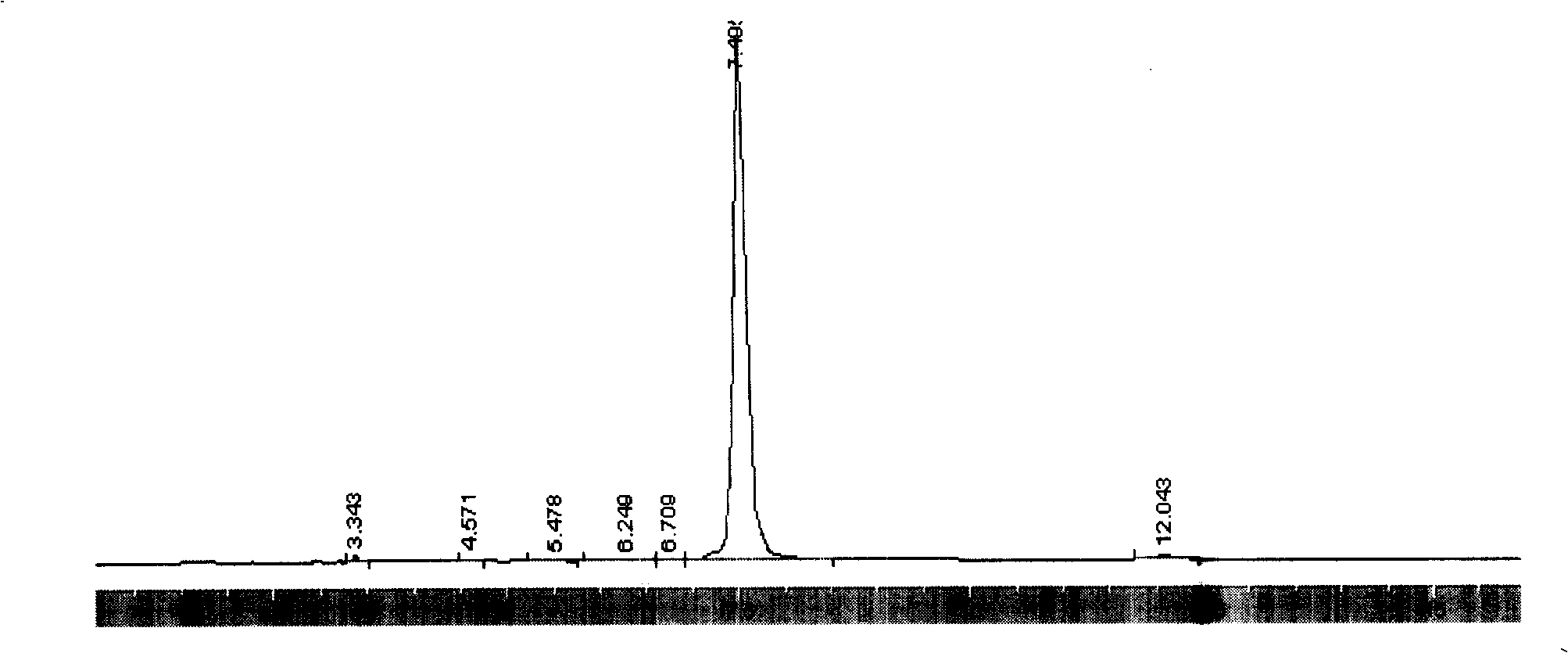
[0028] Embodiment 3: in 250ml there-necked flask, add 8.0 gram solid phosgene, 10 gram purity is the mixed solution (mass ratio is 5: 95) of 9-99% 9-fluorenemethanol and 100ml toluene and normal hexane, at-5 ℃ Stir, add 2.5 g of diethylamine, react for 2 hours, heat up to 25-30°C, stir for 2 hours, filter to remove solids. The obtained filtrate was cooled in an ice bath for 3 hours, after Fmoc-Cl was fully crystallized and separated out, filtered and dried to obtain 10.77 grams of 9-fluorenylmethyl chloroformate product with a purity of 98.47% (the chromatogram is shown in image 3 ), yield 81.40%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com