Fluorescent probe for detecting nitrogen monoxide and preparation method thereof
A nitric oxide, fluorescent probe technology, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., to achieve the effect of reducing interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Probes 1 preparation method
[0037] Step 1. In a 25mL round bottom flask, add 0.5g of 4-amino-1,8-naphthoic anhydride (1.0eq) and 6.0mL of N,N-dimethylformamide, and add 2-(2-amino Ethoxy)ethanol (1.2eq) totaled 0.282mL, heated and stirred at 90°C for 8h; cooled to room temperature, solids precipitated after adding saturated brine, centrifuged and dried to obtain the intermediate with the following structure:
[0038]
[0039] Step 2: Dissolve 0.1 g of the above intermediate in 2 mL of pyridine, add 0.040 mL of acetic anhydride, stir at room temperature and react overnight, and dry the organic solvent pyridine by rotary evaporation; then add 10 mL of water, and extract twice with 10 mL of ethyl acetate; The organic phase was dried with anhydrous sodium sulfate for 1 h, the organic solvent was dried by rotary evaporation, and then separated by column chromatography (ethyl acetate / dichloromethane=1 / 10) to obtain an orange-red solid with the following struc...
Embodiment 2
[0047] Example 2: Probes 1 chemical properties
[0048] (1) Preparation steps of the test solution:
[0049] In a 10mL colorimetric tube, add 1.0mL 3,3-dimethylglutaric acid-sodium hydroxide buffer solution (pH 7.40), and then add different volumes of 1.0×10 -3 M's NOC13 standard solution (prepared nitric oxide carrier NOC13, see literature: Hrabie J.A., Klose J.R.J.Org.Chem.1993, 58, 1472-1476.), diluted to the mark with deionized water 3 times, shaken; Add 0.1mL 1.0×10 -3 m 1 The acetonitrile stock solution was shaken again, placed in a water bath at 37°C for 40 minutes, then excited at 438nm, and measured the fluorescence intensity at 540nm. Operate as above, but do not add nitric oxide or other active oxygen probes 1 Solution, for the preparation of blank test solution.
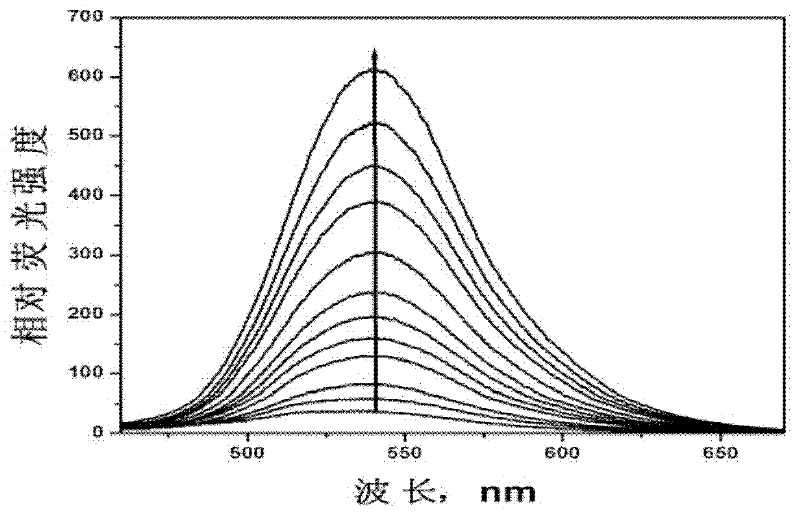
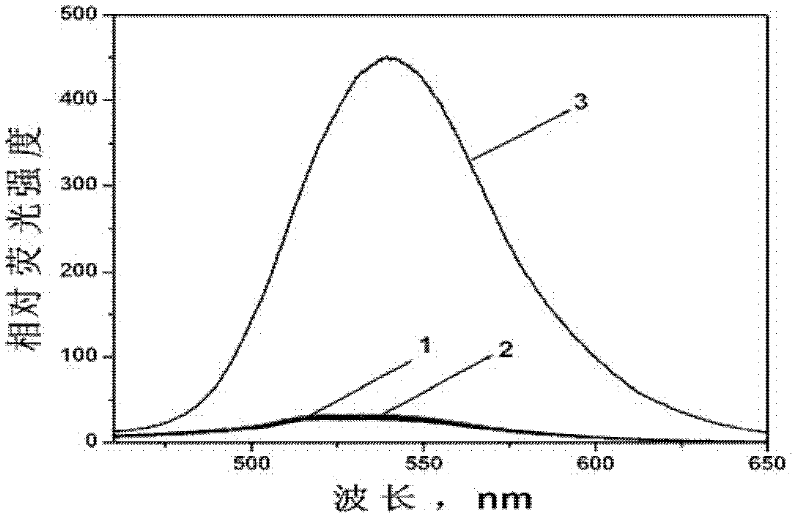
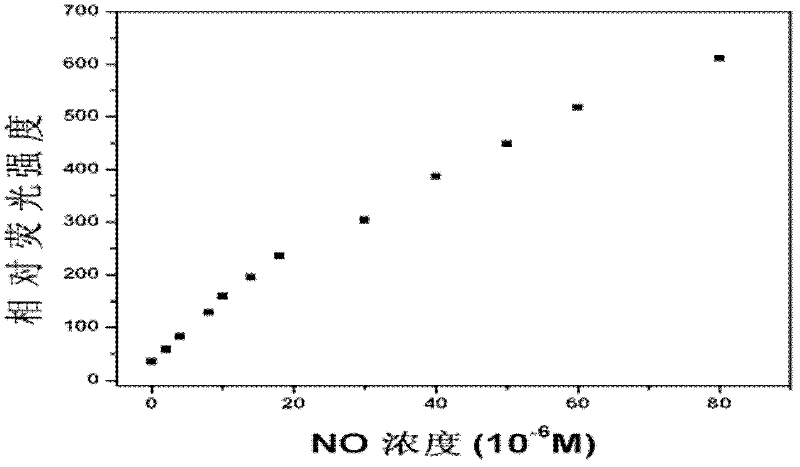
[0050] (2) Probe in aqueous solution 1 Spectral characteristics and detection performance.
[0051] figure 1 is the probe 1 Fluorescence response to nitric oxide in aqueous solution under neutra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com