Preparation method of novel hyperbranched antibacterial peptide polymer
An antibacterial peptide and polymer technology, applied in the field of preparation of antibacterial biological materials, can solve problems such as inapplicability to systemic medication, strong hemolysis, etc., achieve good antibacterial effect and biocompatibility, simple preparation method and low synthesis cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
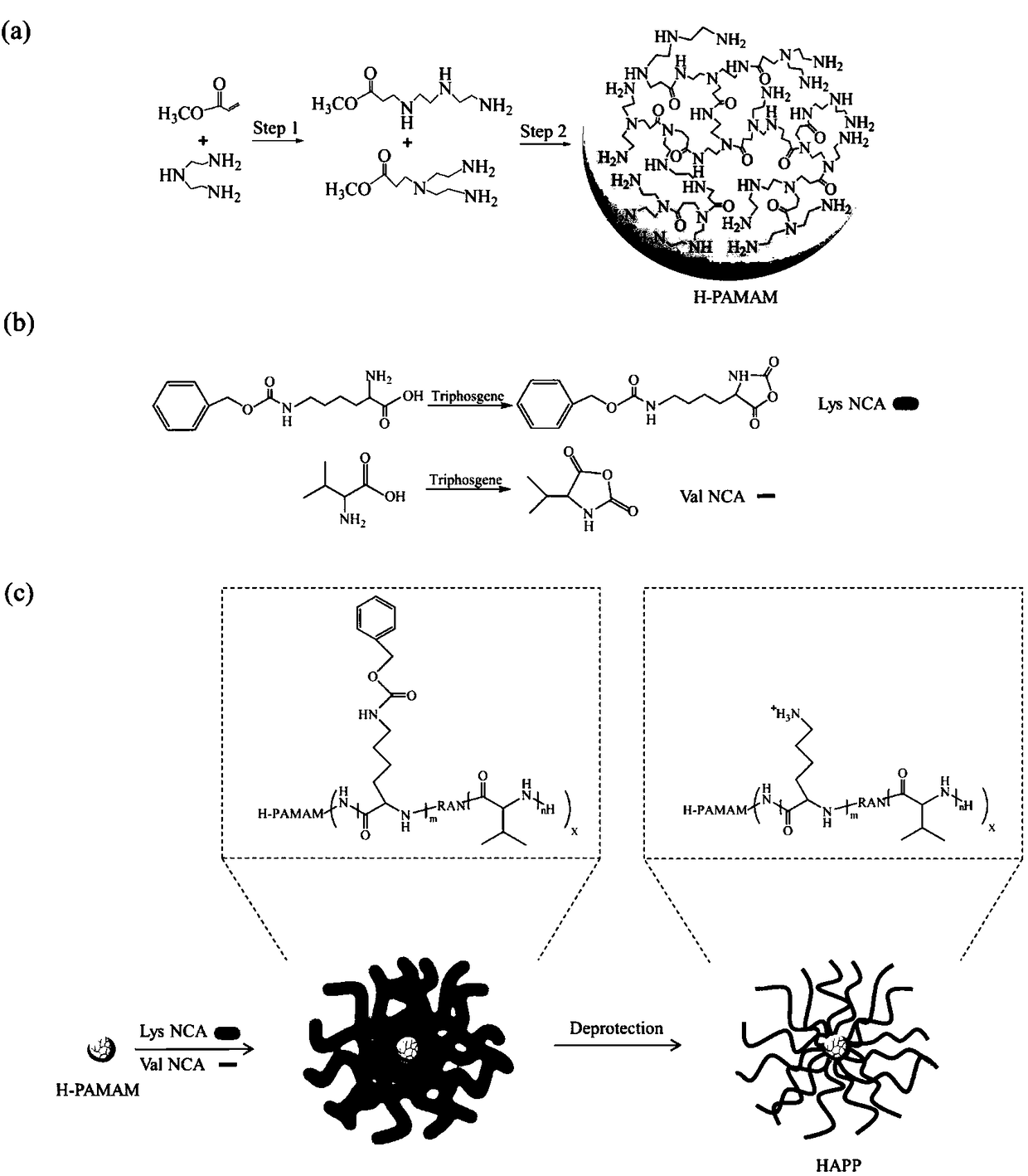
[0024] like figure 1 Shown, a kind of preparation method of novel hyperbranched antimicrobial peptide polymer, the steps are:
[0025] 1) Synthesis of amino-terminated hyperbranched polyamidoamine (H-PAMAM): take a certain amount of diethylenetriamine (DETA) and dissolve it in anhydrous methanol, pass nitrogen protection, and add magnetic stirring. Add a certain amount of methacrylate (MA) dropwise to the above solution through a syringe, wherein the molar ratio of feeding is DETA:MA=1.2:1. Subsequently, the reaction device was transferred to an ice-water bath, stirred for 2 h, and then moved to room temperature for 48 h. Finally, the system was transferred to an oil bath, followed by vacuuming at 60°C / 80°C / 100°C / 120°C for 1h / 1h / 1h / 1.5h respectively to remove the solvent and unreacted monomers. After the reaction, a large amount of glacial ether was used to precipitate the product three times to obtain a yellow viscous product, namely H-PAMAM.
[0026] 2), Synthesis of Lysi...
Embodiment 2
[0029] A kind of preparation method of novel hyperbranched antimicrobial peptide polymer, the steps are:
[0030] 1) Synthesis of amino-terminated hyperbranched polyamidoamine (H-PAMAM): Take a certain amount of diethylenetriamine (DETA) and dissolve it in anhydrous methanol, pass nitrogen protection, and add magnetic stirring. Add a certain amount of methacrylate (MA) dropwise to the above solution through a syringe, wherein the molar ratio of feeding is DETA:MA=1.2:1. Subsequently, the reaction device was transferred to an ice-water bath, stirred for 2 h, and then moved to room temperature for 48 h. Finally, the system was transferred to an oil bath, followed by vacuuming at 60°C / 80°C / 100°C / 120°C / 140°C for 1h / 1h / 1h / 1.5h / 2h respectively to remove solvent and unreacted monomer. After the reaction, a large amount of glacial ether was used to precipitate the product three times to obtain a yellow viscous product, namely H-PAMAM.
[0031] 2), Synthesis of Lysine-N-Carboxylic A...
Embodiment 3
[0034] A kind of preparation method of novel hyperbranched antimicrobial peptide polymer, the steps are:
[0035] 1) Synthesis of amino-terminated hyperbranched polyamidoamine (H-PAMAM): Take a certain amount of diethylenetriamine (DETA) and dissolve it in anhydrous methanol, pass nitrogen protection, and add magnetic stirring. Add a certain amount of methacrylate (MA) dropwise to the above solution through a syringe, wherein the molar ratio of feeding is DETA:MA=1.2:1. Subsequently, the reaction device was transferred to an ice-water bath, stirred for 2 h, and then moved to room temperature for 48 h. Finally, the system was transferred to an oil bath, followed by vacuuming at 60°C / 80°C / 100°C / 120°C for 1h / 1h / 1h / 1.5h respectively to remove the solvent and unreacted monomers. After the reaction, a large amount of glacial ether was used to precipitate the product three times to obtain a yellow viscous product, namely H-PAMAM.
[0036] 2), Synthesis of Lysine-N-Carboxylic Anhydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com