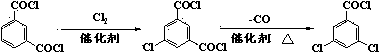
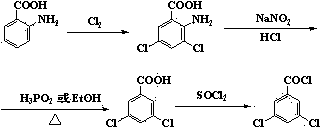
Method for synthesizing 3,5-dichlorobenzoyl chloride
A technology for the synthesis of dichlorobenzoyl chloride and its synthesis method, which is applied in the field of synthesis of 3,5-dichlorobenzoyl chloride, an intermediate of agricultural chemicals, and can solve the problems of large amount of hazardous chemicals, low production safety, three wastes and corrosion and other issues, to achieve the effects of high production safety, high product yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Heat 140.5g (1.0mol) of benzoyl chloride to 130°C, add 176g (2.2mol) of sulfur trioxide dropwise, and control the dropping for 2 hours. During the dropwise addition, the temperature was gradually raised to 170°C, and continued to be raised to 220°C after the dropwise addition and kept for 16 hours. After cooling to 120°C, 2.0 g of catalyst 4-dimethylaminopyridine (DMAP) and 60 g of mixed xylene were added. Within 1 hour, 218 g (2.2 mol) of triphosgene was added in 6 batches, and the reaction was kept for 3 hours. The solvent was recovered by distillation under reduced pressure until the inner temperature reached 150°C. At a temperature of 175° C., chlorine gas is passed into the reaction solution under the liquid surface at a rate of 0.4 g / min, and sulfur dioxide gas escapes immediately. After 2.5 hours of reaction, no more sulfur dioxide was released. Cool to 50°C and distill under reduced pressure to obtain 182g of crude product with a yield of 86.8%, ...
Embodiment 2
[0034] Example 2 Heat 140.5g (1.0mol) of benzoyl chloride to 140°C, add 176g (2.2mol) of sulfur trioxide dropwise, and control the dropping for 2 hours. During the dropwise addition, the temperature was gradually raised to 170°C, and continued to be raised to 220°C after the dropwise addition and kept for 16 hours. After cooling to 100° C., 1.1 g of a catalyst triethylenediamine (DABCO) and 50 g of chlorobenzene were added. Within 1 hour, 218 g (2.2 mol) of triphosgene was added in 6 batches, and the reaction was kept for 4 hours. The solvent was recovered by distillation under reduced pressure until the inner temperature reached 150°C. Under the condition of heat preservation, chlorine gas is fed under the liquid surface of the reaction solution at a rate of 0.4 g / min, and sulfur dioxide gas escapes immediately. After 2.0 hours of reaction, no sulfur dioxide is released. Cooled to 50°C and distilled under reduced pressure to obtain 179g of crude product with a yield of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com