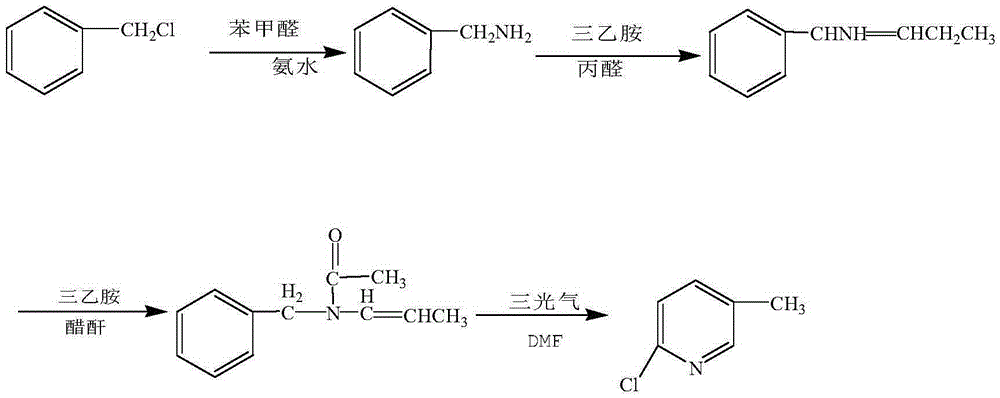
Preparation method of 2-chloro-5-picoline
A technology of methyl pyridine and benzyl chloride, applied in the field of preparation of 2-chloro-5-methyl pyridine, can solve the problems of difficult industrialization, many by-products, complicated operation, etc., and achieves reduction of production processing steps and improved reaction Selectivity, the effect of reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of compound b benzylamine
[0035] In a 10000L stainless steel reaction kettle, put 2000kg of water and pass through ammonia gas, the detection concentration reaches 25-30%, add or recover 2000kg of benzyl chloride and 1800kg of benzaldehyde, heat up to 60°C in a closed system, and control the pressure at 2 atm, keep warm for 6-8 hours, HLC traces benzyl chloride ≤ 0.1%, that is, the reaction is complete. After the reaction, the solution cools down to 20-25°C and stratifies. Put it into another 10000L reaction kettle, use 1600kg of 30% hydrochloric acid, adjust PH=1, raise the temperature to 60°C, and separate layers at rest. The oil layer is benzaldehyde, which can be applied mechanically, and the water layer is benzylamine hydrochloride. 1600kg of 40% liquid caustic soda, adjusted to PH=10, static layering, to obtain crude benzylamine, which was then collected by vacuum distillation at 90-102°C / 10mmHg to obtain 1573.3kg of compound benzylamine, GC≥99.2...
Embodiment 2
[0043] The preparation of compound b benzylamine
[0044] In a 10000L stainless steel reaction kettle, put 2000kg of water and pass through ammonia gas, and the detection concentration will reach 25-30%. 1.5 atm, keep warm for 6-8 hours, HLC traces benzyl chloride ≤ 0.1%, that is, the reaction is complete, after the reaction, the solution cools down to 20-25°C and stratifies, and 635kg of caustic soda is added to the water layer, and the next batch of ammonia gas is put into the oil layer. Transfer to another 10000L reaction kettle, use 1600kg of 30% hydrochloric acid, adjust PH=1, heat up to 55°C, and separate into layers, the oil layer is benzaldehyde, and the water layer is benzylamine hydrochloride. At 30°C, Add 1600kg of 40% liquid caustic soda, adjust the pH to 10-11, and separate layers at rest to obtain crude benzylamine. The crude benzylamine is then collected by vacuum distillation at 90-102°C / 10mmHg to obtain 1573.3kg of compound benzylamine, GC≥99.2%, molar yield ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com