Preparation method of ponazuril
A technology of methyl and trifluoromethylthiophenoxy, which is applied in the field of preparation of the anti-coccidiosis drug Patopazuril, can solve the problems of low stability, unfavorable industrial production, cumbersome operation, etc., and achieve easy scale The effect of chemical industry production, low production cost and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
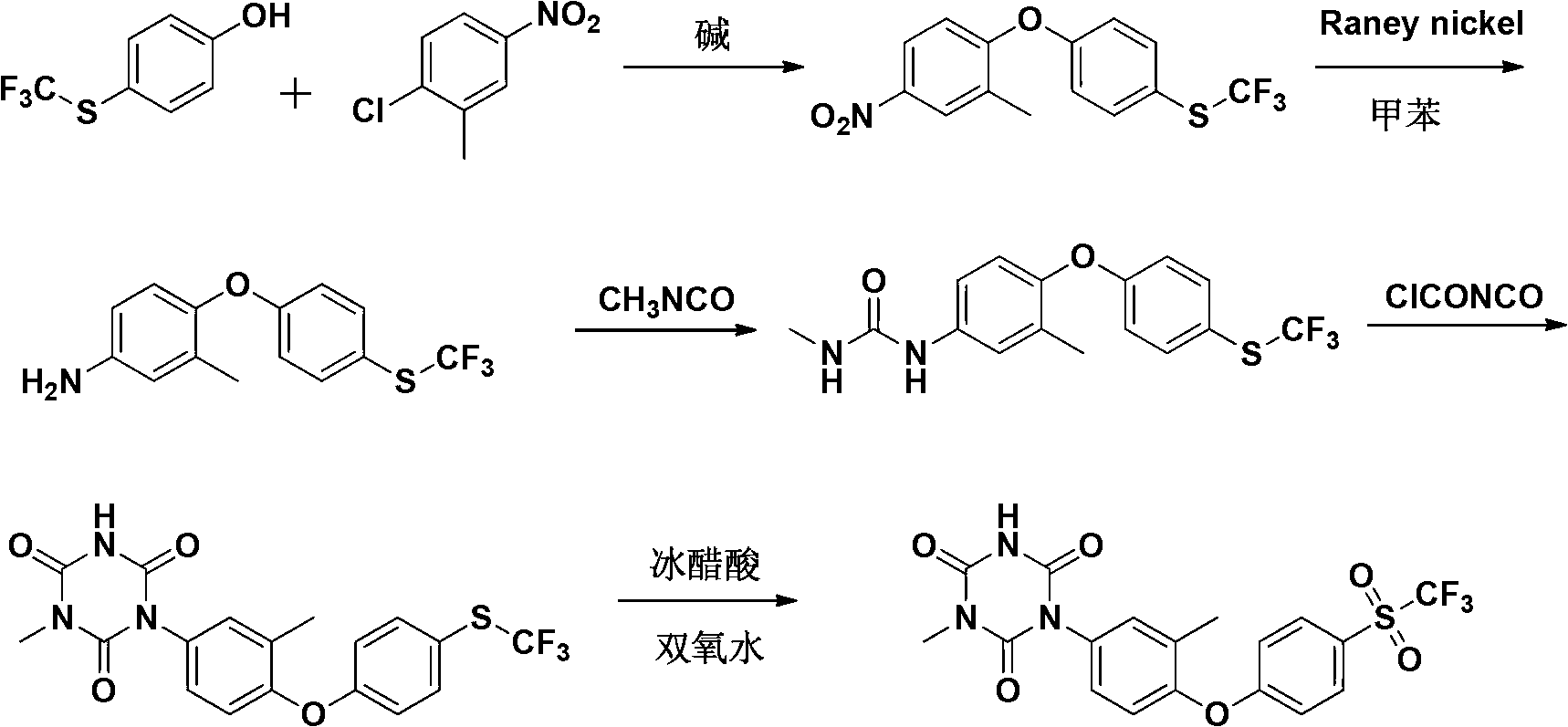
[0055] A preparation method of Patopazuril, comprising the steps of:
[0056] (1) Synthesis of 3-methyl-4-(4-trifluoromethylthiophenoxy)-nitrobenzene:
[0057] P-trifluoromethylthiophenol (194g, 1mol), anhydrous K 2 CO 3 (179g, 1.3mol) and dimethylsulfoxide (DMSO) (390g, 5mol) were put into a 2000mL four-neck round bottom flask, heated to 90°C, stirred for 20min, and 2-chloro-5-nitro was added dropwise within 1h Toluene (171g, 1mol) in dimethyl sulfoxide (314g, 4mol) solution, after dripping, react at 140°C for 6h, TLC detects that the reaction is complete, concentrate under reduced pressure, add ice water (600mL) to the concentrated solution, cool and precipitate , filtered, the filter cake was washed twice with saturated brine, dried in vacuo, recrystallized from petroleum ether to obtain 302 g of light yellow crystals, yield 92%, melting point (m.p): 60~62°C;
[0058] (2) Synthesis of 3-methyl-4-(4-trifluoromethylthiophenoxy)-aniline:
[0059] The compound 3-methyl-4-(4...
Embodiment 2
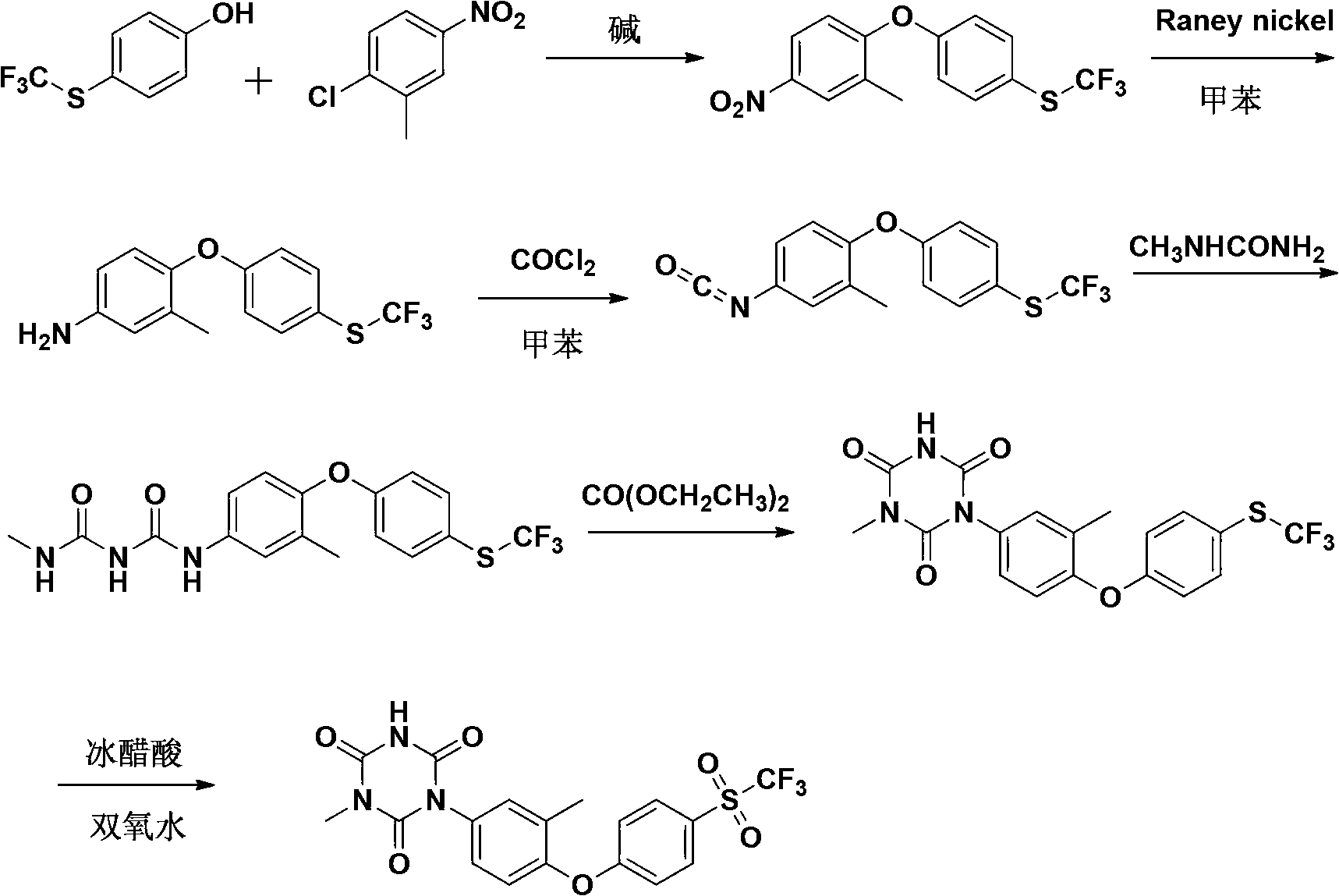
[0067] A preparation method of Patopazuril, comprising the steps of:
[0068] (1) Synthesis of 3-methyl-4-(4-trifluoromethylthiophenoxy)-nitrobenzene:
[0069] Put p-trifluoromethylthiophenol (194g, 1mol), NaOH (44g, 1.1mol) and dimethyl sulfoxide (DMSO) (390g, 5mol) into a 2000mL four-neck round bottom flask, heat to 80°C, and stir For 20 minutes, add a solution of 2-chloro-5-nitrotoluene (188g, 1.1mol) in dimethyl sulfoxide (314g, 4mol) dropwise within 1 hour. After the drop, react at 140°C for 5 hours, TLC detects that the reaction is complete, and depressurizes Concentrate, add ice water (600mL) to the concentrated solution, cool to precipitate the precipitate, filter, wash the filter cake twice with saturated brine, dry in vacuo, recrystallize from petroleum ether to obtain 306g of light yellow crystals, yield 93%, m.p.: 60 ~62°C;
[0070] (2) Synthesis of 3-methyl-4-(4-trifluoromethylthiophenoxy)-aniline:
[0071] The compound 3-methyl-4-(4-trifluoromethylthiophenoxy)...
Embodiment 3
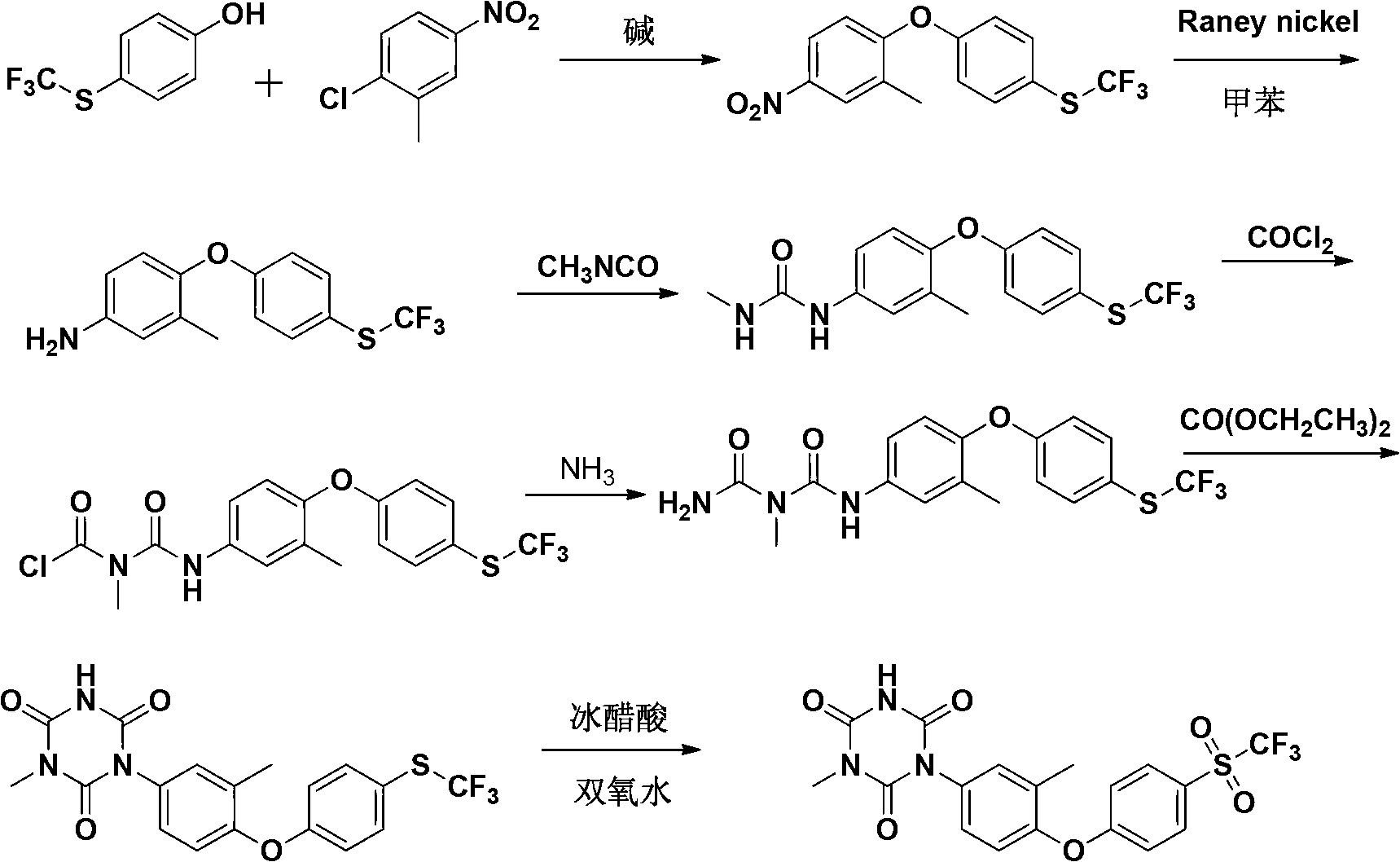
[0079] A preparation method of Patopazuril, comprising the steps of:
[0080] (1) Synthesis of 3-methyl-4-(4-trifluoromethylthiophenoxy)-nitrobenzene:
[0081] Put p-trifluoromethylthiophenol (194g, 1mol), KOH (62g, 1.1mol) and dimethyl sulfoxide (DMSO) (468g, 6mol) into a 2000mL four-necked round-bottomed flask, heated to 80°C, Stir for 20min, add dropwise a solution of 2-chloro-5-nitrotoluene (188g, 1.1mol) in dimethyl sulfoxide (314g, 4mol) within 1h. Concentrate under reduced pressure, add ice water (600mL) to the concentrated solution, cool to precipitate the precipitate, filter, wash the filter cake twice with saturated brine, dry in vacuo, and recrystallize from petroleum ether to obtain 302g of light yellow crystals, with a yield of 92%, m.p.: 60~62℃;
[0082] (2) Synthesis of 3-methyl-4-(4-trifluoromethylthiophenoxy)-aniline:
[0083] The compound 3-methyl-4-(4-trifluoromethylthiophenoxy)-nitrobenzene (302g, 0.92mol) prepared in step (1), aluminum powder (86.4g, 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com