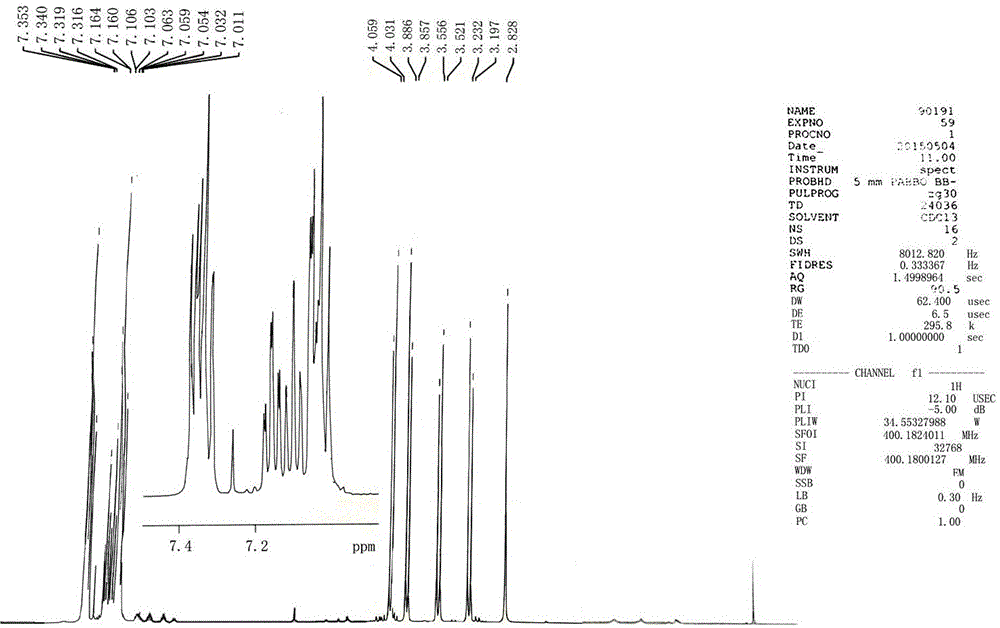
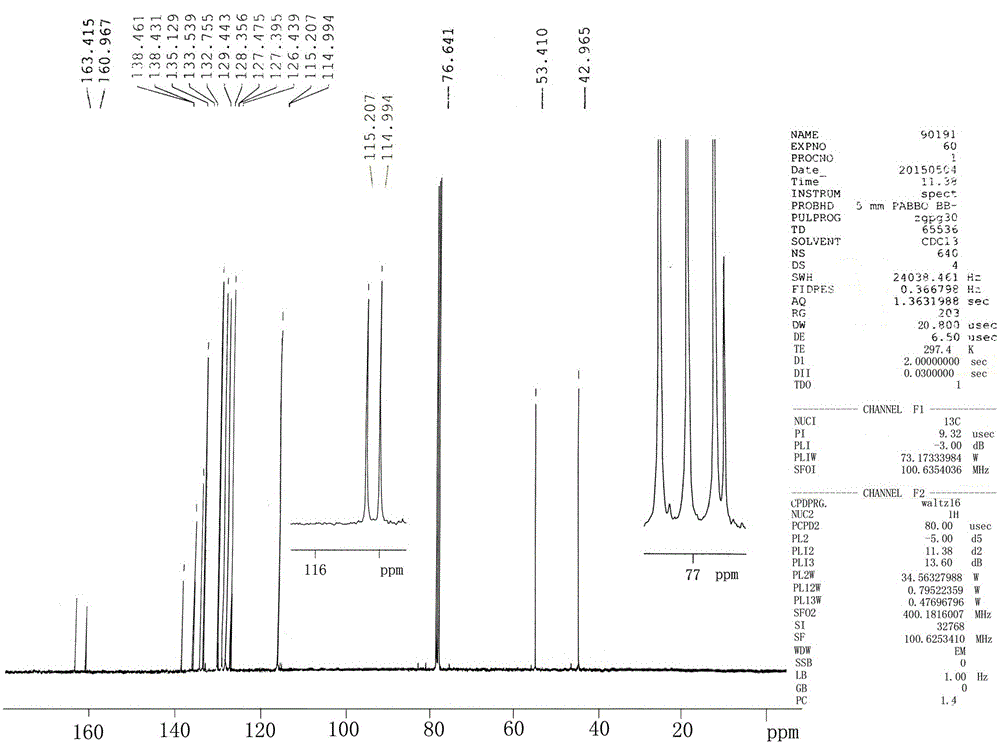
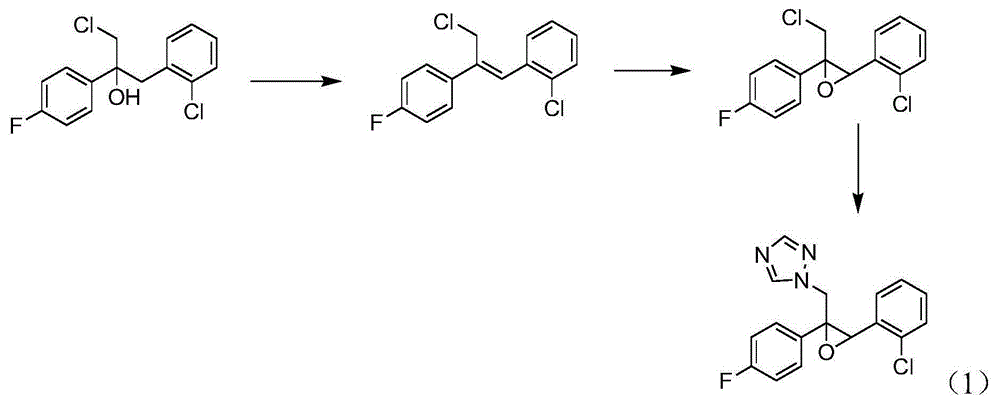
Epoxiconazole intermediate 1-chloro-3-(2-chlorophenyl)-2-(4-fluorophenyl)-2-propanol synthesis process
A synthesis process and a technology for intermediates, applied in the field of fine chemicals, can solve the problems of difficult industrial production, seldom application of aryl ketone compounds, poor safety, etc., and achieve low equipment consumption and energy consumption, easy solvent recovery, and easy operation. safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This embodiment is the synthesis of 1-chloro-3-(2-chlorobenzene)-2-(4-fluorobenzene)-2-propanol, and the specific process is as follows:
[0028] Put 17.5g of magnesium chips (0.729mol, 99%, 1.2eq.) into the 1000mL four-necked bottle replaced by nitrogen, put 40g of diethoxymethane into it, and protect the whole process with nitrogen at a temperature of 20-25°C; 10g (0.06mol, 99%, 0.1eq.) of o-chlorobenzyl chloride was added dropwise in the middle, and the dropwise addition was completed in 5 minutes, and the temperature was naturally raised, and the reaction was initiated; Add dropwise the remaining 87.5g of o-chlorobenzyl chloride (0.54mol, 99%, 0.9eq.); after the dropwise addition (about 1h), keep the temperature at 10-15°C for 1h to obtain the Grignard reagent, and take a sample for analysis.
[0029] After the Grignard reagent was prepared, the Grignard reagent was transferred into a 2000mL four-neck flask under nitrogen protection; the Grignard reagent was cooled ...
Embodiment 2
[0032] This example is the synthesis of 1-chloro-3-(2-chlorobenzene)-2-(4-fluorobenzene)-2-propanol, the difference from Example 1 is that the reaction solvent is toluene and diethoxy The mixed solvent of methyl methane, the weight ratio of toluene, diethoxymethane and o-chlorobenzyl chloride is 2.6:1.3:1, and the molar ratio of magnesium and o-chlorobenzyl chloride is 1.2:1.
Embodiment 3
[0034] This example is the synthesis of 1-chloro-3-(2-chlorobenzene)-2-(4-fluorobenzene)-2-propanol, the difference from Example 1 is that the reaction solvent is toluene and diethoxy The mixed solvent of methyl methane, the weight ratio of toluene, diethoxymethane and o-chlorobenzyl chloride is 1.3:2.6:1, and the molar ratio of magnesium and o-chlorobenzyl chloride is 1.2:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com