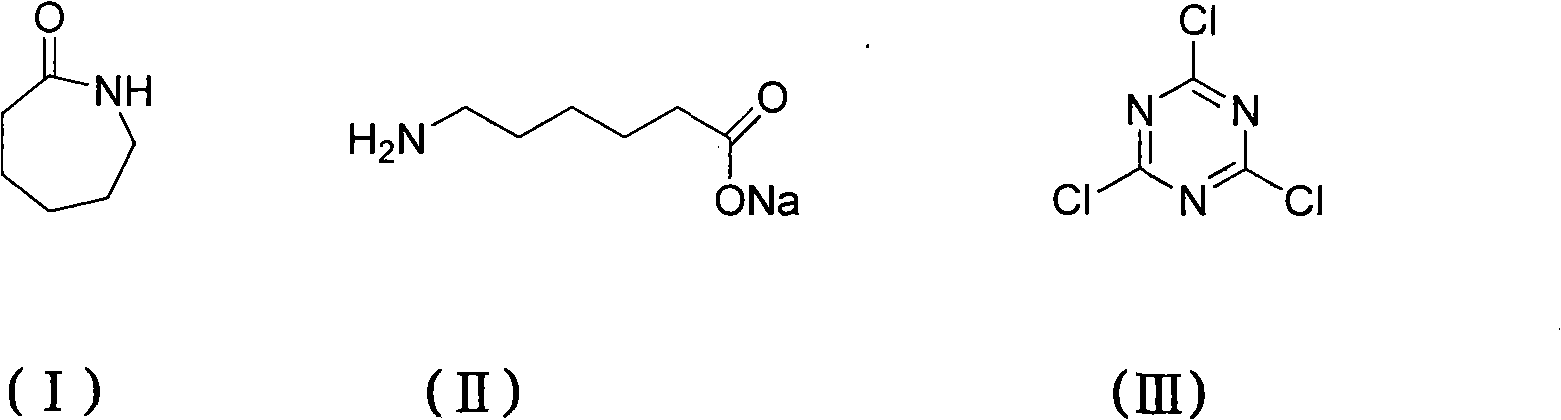
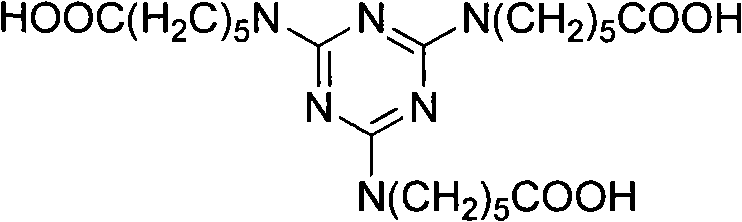
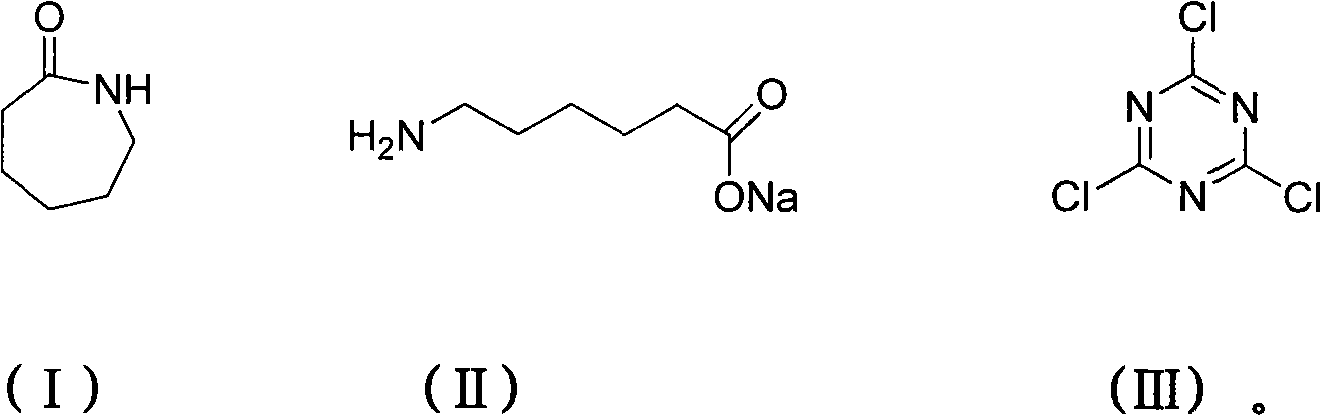
Method for preparing 2,4,6-tri(amino caproyl)-1,3,5-triazine
A technology of aminocaproic acid and carboxylate, which is applied in 2 fields, can solve the problems such as not being found, and achieve the effect of simple and easy reaction operation, short time and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add sodium hydroxide (32.8g, 0.82mol), caprolactam (92.6g, 0.82mol) and 400mL water respectively in a 1000mL three-neck round bottom flask equipped with a magnet, reflux condenser and thermometer, start stirring and control the temperature at 60 °C for 30 minutes. After the reaction, cyanuric chloride (50.4 g, 0.27 mol) was added, the temperature was raised to 70° C., and maintained for 120 minutes, during which the reaction progress was monitored by TLC, and ethanol was used as a developing solvent. Cool down to 30°C, add concentrated hydrochloric acid (82g, 0.82mol) with a concentration of 36.5%, precipitate a wet cake-like white solid, stir for 30 minutes, filter with suction, and dry at 80°C to obtain 54 grams of product, yield 88.7%. m.p.181-183℃.IR: v=3300, 2942, 2364, 1710, 1567, 1413, 1344, 1257, 1186, 843, 785, 742cm -1 . 1 H NMR (DMSO, 300MHz): δ=1.25(t, J=6.9Hz, 6H), 1.37-1.60(m, 12H), 2.14-2.28(m, 12H), 6.20-6.69(t, J=6.3Hz , 3H).Anal.Calcd for C 21 h 3...
Embodiment 2
[0023] Add sodium hydroxide (43.7g, 1.1mol), caprolactam (92.6g, 0.82mol) and 400mL water respectively in a 1000mL three-neck round bottom flask equipped with a magnet, reflux condenser and thermometer, start stirring and control the temperature at 70 °C for 40 minutes. After the reaction, cyanuric chloride (50.4 g, 0.27 mol) was added, the temperature was raised to 75° C., and maintained for 100 minutes, during which the reaction progress was monitored by TLC, and ethanol was used as a developing solvent. Cool down to 35°C, add concentrated hydrochloric acid (164g, 1.64mol) with a concentration of 36.5%, precipitate a wet cake-like white solid, stir for 50 minutes, filter with suction, and dry at 80°C to obtain 54 g of the product with a yield of 88.4%. m.p.181-183℃.IR: v=3300, 2942, 2364, 1710, 1567, 1413, 1344, 1257, 1186, 843, 785, 742cm -1 . 1 H NMR (DMSO, 300MHz): δ=1.25(t, J=6.9Hz, 6H), 1.37-1.60(m, 12H), 2.14-2.28(m, 12H), 6.20-6.69(t, J=6.3Hz , 3H).Anal.Calcd for C...
Embodiment 3
[0025] Add sodium hydroxide (54.6g, 1.37mol), caprolactam (92.6g, 0.82mol) and 400mL water respectively into a 1000mL three-neck round bottom flask equipped with a magnet, reflux condenser and thermometer, start stirring and control the temperature at 65 °C for 30 minutes. After the reaction, cyanuric chloride (50.4 g, 0.27 mol) was added, the temperature was raised to 75° C., and maintained for 90 minutes, during which the reaction progress was monitored by TLC, and ethanol was used as a developing solvent. Cool down to 40°C, add concentrated hydrochloric acid (219g, 2.19mol) with a concentration of 36.5%, precipitate a wet cake-like white solid, stir for 60 minutes, filter with suction, and dry at 80°C to obtain 53 g of the product, with a yield of 87%. m.p.181-183℃.IR: v=3300, 2942, 2364, 1710, 1567, 1413, 1344, 1257, 1186, 843, 785, 742cm -1 . 1 H NMR (DMSO, 300MHz): δ=1.25(t, J=6.9Hz, 6H), 1.37-1.60(m, 12H), 2.14-2.28(m, 12H), 6.20-6.69(t, J=6.3Hz , 3H).Anal.Calcd for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com