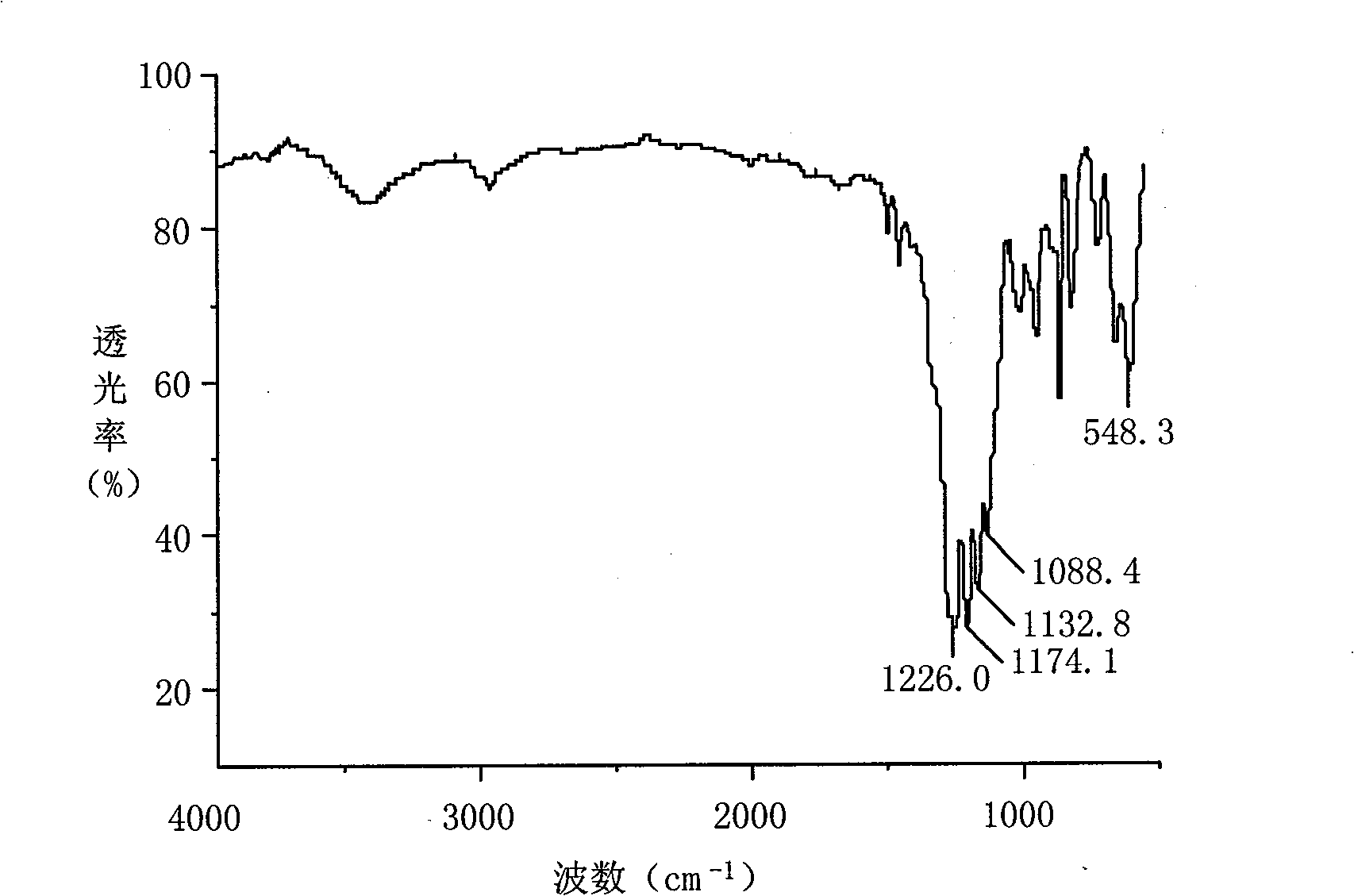
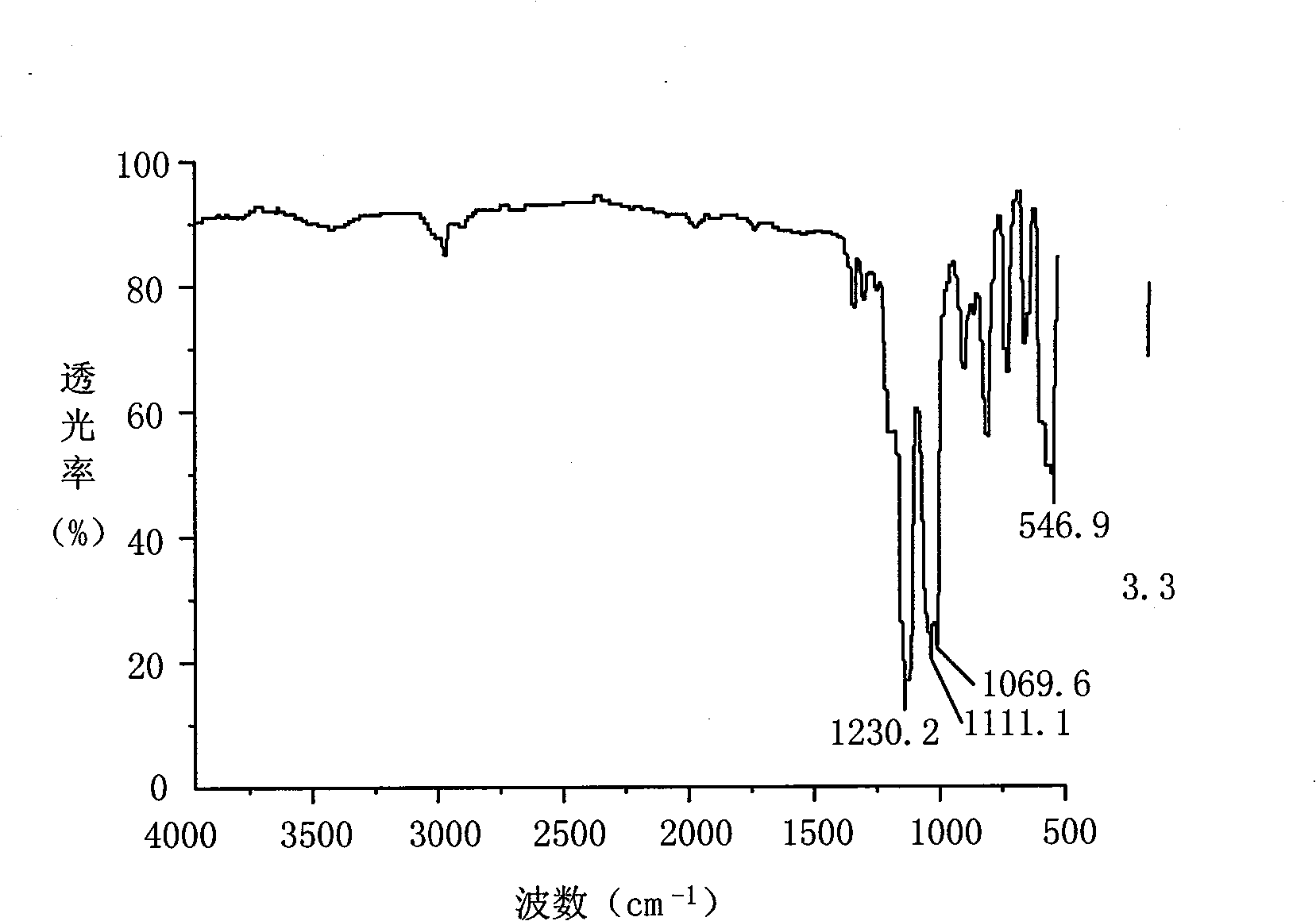
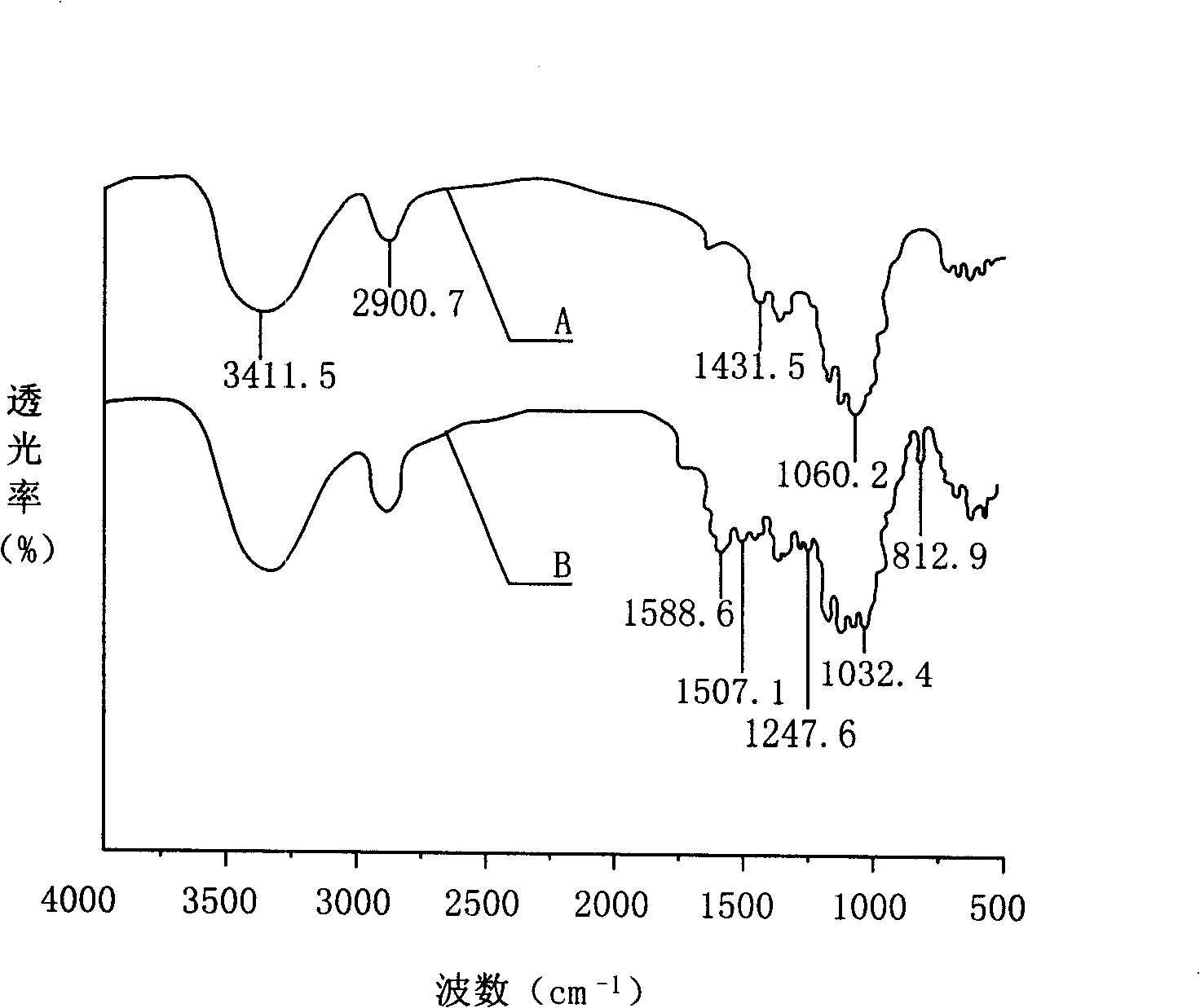
Fluoride alkoxycyclotriphosphazene derivative, preparation and use thereof
A technology of fluorine-containing alkoxycyclotriphosphazene and derivatives, which is applied in the fields of polymer synthesis and textile finishing, and achieves the effects of wide application fields and excellent flame retardant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Synthesis of Fluorinated Alkoxycyclotriphosphazene Derivatives:
[0021] Add 1000 mL of tetrahydrofuran and 232 g of octafluoropentanol into a 2L three-necked flask equipped with a mechanical stirring device, and stir to dissolve. Weigh 26g of sodium metal, cut into small particles, and add 1g of each batch to the octafluoropentanol / tetrahydrofuran solution in batches. After the previous batch of sodium metal disappears completely, add the second batch until all the sodium metal is completely Add, and continue to stir for 1 hour, so that the reaction of the sodium metal is basically complete, and a tetrahydrofuran solution of sodium octafluoropentoxide is obtained.
[0022] Add 500mL of tetrahydrofuran and 58g of hexachlorocyclotriphosphazene into a 3L three-neck flask equipped with a mechanical stirring device, a reflux condenser and a constant pressure addition funnel, and stir until the hexachlorocyclotriphosphazene is completely dissolved. The sodium octafluoropent...
Embodiment 2
[0031] Synthesis of Fluorinated Alkoxycyclotriphosphazene Derivatives:
[0032] Add 1000mL of tetrahydrofuran and 264g of tetrafluoropropanol into a 2L three-necked flask equipped with a mechanical stirring device, and stir to dissolve. Weigh 52g of sodium metal, cut into small particles, and add 1g per batch to the tetrafluoropropanol / tetrahydrofuran solution in batches. After the previous batch of sodium metal disappears completely, add the second batch until all the sodium metal is completely After adding, continue to stir for 1h to make the sodium metal completely react to obtain a tetrahydrofuran solution of sodium tetrafluoropropoxide.
[0033] Add 700mL of tetrahydrofuran and 116g of hexachlorocyclotriphosphazene into a 3L three-necked flask equipped with a mechanical stirring device, a reflux condenser and a constant pressure addition funnel, and stir until the hexachlorocyclotriphosphazene is completely dissolved. The sodium tetrafluoropropoxide / tetrahydrofuran solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| smoldering time | aaaaa | aaaaa |
| Continue burning | aaaaa | aaaaa |
| whiteness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com