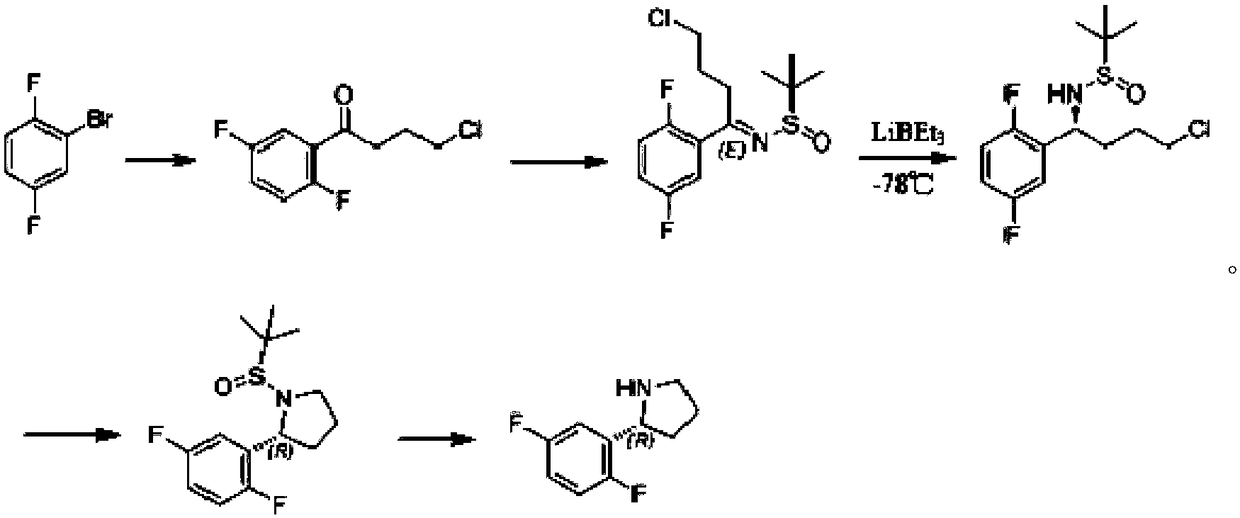
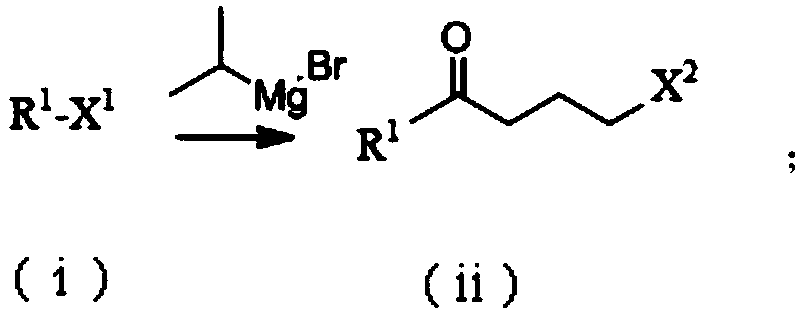
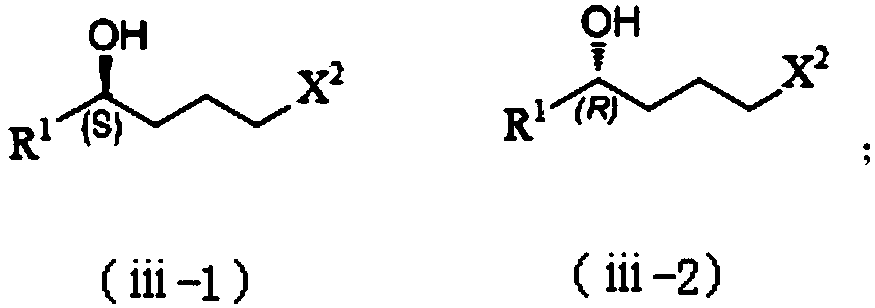Synthesis process of chiral pyrrolidine and intermediates
A synthesis process and pyrrolidine technology, which is applied in the synthesis process and intermediate field of chiral pyrrolidine, can solve the problems of large demand, high production cost and high price of lithium triethyl borohydride, and achieve easy control and industrialization , Reduction of reaction steps, high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] This example is used to illustrate the synthesis process of Route 1.
[0067] Step 1: Synthesis of the compound of formula (A) (4-chloro-1-(2,5-difluorophenyl)-1-butanone)
[0068]
[0069] Add isopropylmagnesium chloride (2M THF solution, 60ml, 120mmol) in a 500ml three-necked flask, cool to 0 degrees, start to add dropwise a tetrahydrofuran (150ml) solution of 2,5-difluorobromobenzene (19.3g, 100mmol), After the dropwise addition, react at room temperature for 2h; then, control the temperature below 20°C, add dropwise a solution of N-methoxy-N-methyl-4-chlorobutanamide (16.5g, 100mmol) in tetrahydrofuran (50ml), drop After the addition, react at room temperature for 16 hours; add 50ml of hydrochloric acid with an equivalent concentration of 2N to the reaction mixture dropwise, stir for 30min, separate the liquids, extract the aqueous phase twice with 100ml of ethyl acetate (EA), combine the organic phases, and use It was washed with 50 ml of saturated brine, dried...
Embodiment 2
[0084] This example is used to illustrate the synthesis process of Route 1.
[0085] Step 1: the synthesis of formula (E) compound
[0086]
[0087] Add isopropylmagnesium chloride (2M THF solution, 60ml, 120mmol) in a 500ml three-necked flask, cool to 0°C, start to drop a solution of 5-fluoro-3-bromopyridine (17.5g, 100mmol) in tetrahydrofuran (150ml), After the dropwise addition, react at room temperature for 2h; then, control the temperature below 20°C, add dropwise a solution of N-methoxy-N-methyl-4-chlorobutanamide (16.5g, 100mmol) in tetrahydrofuran (50ml), drop After the addition, react at room temperature for 16 h; then, add 50 ml of hydrochloric acid with an equivalent concentration of 2N dropwise to the reaction mixture, stir for 30 min, separate the liquids, extract the aqueous phase twice with 100 ml of ethyl acetate (EA), combine the organic phases, It was washed with 50 ml of saturated brine, dried over anhydrous sodium sulfate, and the organic solvent was re...
Embodiment 3
[0100] This example is used to illustrate the synthesis process of Route 1.
[0101] The difference from Example 1 is that in step 3, methanesulfonyl chloride was replaced by p-toluenesulfonyl chloride, that is, a dichloromethane solution (50 ml) of p-toluenesulfonyl chloride (19.0 g, 99.6 mmol) was added dropwise to the raw material.
[0102] In this embodiment, the yield of the product is 76%, and the ee% is 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com