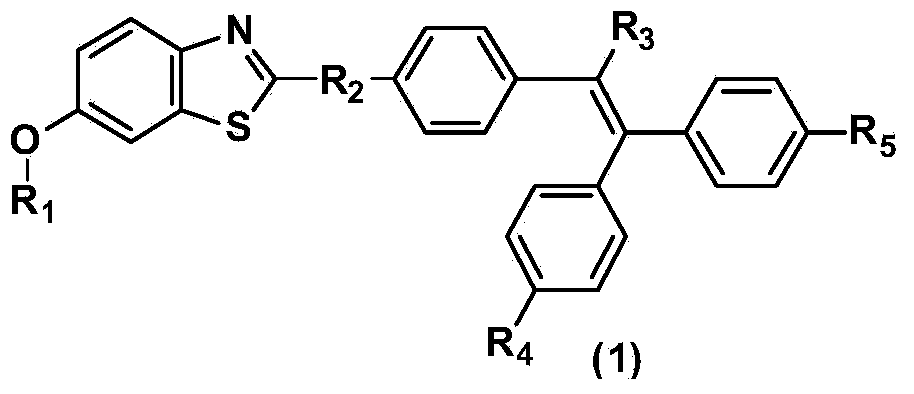
Benzothiazole derivative containing triphenylethylene or tetraphenylethylene structure and having aggregation-induced emission property and preparation method and application thereof
A technology of aggregation-induced luminescence and tetraphenylethylene, which is applied in the direction of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve the problems of limited luminescent performance of benzothiazole derivatives, etc., and achieve low raw material cost and simple synthesis , the effect of obvious aggregation-induced luminescent properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The synthetic method of above-mentioned benzothiazole derivative, comprises the following steps:
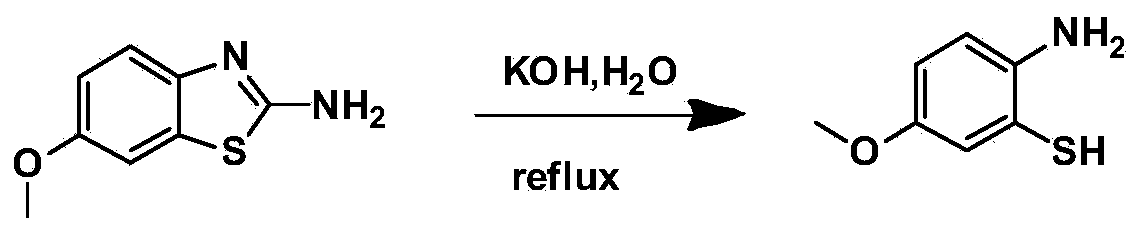
[0019] The first step: 2-amino-6-methoxybenzothiazole ring-opening to generate 2-amino-5-methoxythiophenol
[0020] 2-Amino-6-methoxybenzothiazole is heated and refluxed in water under alkaline conditions to generate 2-amino-5-methoxythiophenol. This step does not use organic solvents, which is economical and environmentally friendly.
[0021]
[0022] Step 2: Synthesis of aromatic aldehydes containing triphenylethylene or tetraphenylethylene
[0023] Using benzophenone derivatives or tristyrene bromide as raw materials, it is synthesized by conventional organic synthesis methods, such as Friedel-Crafts alkylation, amine alkylation, halogenation, Suzuki reaction, Heck reaction, Wittig reaction, etc. Aromatic aldehyde containing triphenylethylene or tetraphenylethylene, its structure is as shown in general formula (4):
[0024]
[0025] where R 2 is a direct single...
Embodiment 1
[0042] Synthesis of 6-methoxy-2-(4-(1,2,3-triphenylethylene)phenyl)benzothiazole:
[0043] (1) Synthetic intermediate 2-amino-5-methoxythiophenol
[0044] Add 2-amino-6-methoxybenzothiazole (9g, 50mmol) into a three-necked flask, and add 50% potassium hydroxide aqueous solution (100g potassium hydroxide, 100g water), heat to reflux, and react for 24h , stop heating, cool to room temperature, suction filter, and the filtrate is neutralized with 30% hydrochloric acid aqueous solution to obtain a tan precipitate, which is repeatedly washed with ultrapure water and dried to obtain 5.4 g of tan powder with a yield of 70%.
[0045]
[0046] (2) Synthesis of intermediate tetraphenyl vinyl aldehyde:
[0047] Add bromotriphenylethylene (6.70g, 20mmol) and p-aldehyde phenylboronic acid (4.50g, 30mmol) into a three-necked flask under Ar atmosphere, then add 80mL of toluene, 25mL of 2mol / L potassium carbonate aqueous solution, tetrabutyl bromide Tribasic ammonium (TBAB) (0.64g, 2.0mm...
Embodiment 2
[0053] Synthesis of 2-(4-(1,2,3-triphenylethylene)phenyl)benzothiazol-6-ol:
[0054] Under an Ar atmosphere, the target product 6-methoxy-2-(4-(1,2,3-triphenylethylene)phenyl)benzothiazole (2g, 4mmol) in Example 1 was added into a three-necked flask, Then add 15mL of dried dichloromethane, stir, cool to 0°C, add boron tribromide (1.00g, 4mmol), react for 12h, add water to quench the excess boron tribromide, and then extract three times with dichloromethane , the organic layer was dried with anhydrous sodium sulfate, the solvent was removed by rotary evaporation under reduced pressure, and purified by silica gel column chromatography, and the eluent was a mixed solvent of dichloromethane and n-hexane with a volume ratio of 1:1. 1.34 g of white product was obtained with a yield of 96%.
[0055]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com