Synthetic methods of 3,6-bis(4-bisfumaroyl aminobutyl)-2,5-diketopiperazine and salt substitute thereof
A bisfumaryl aminobutyl, diketopiperazine technology, applied in 3 fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
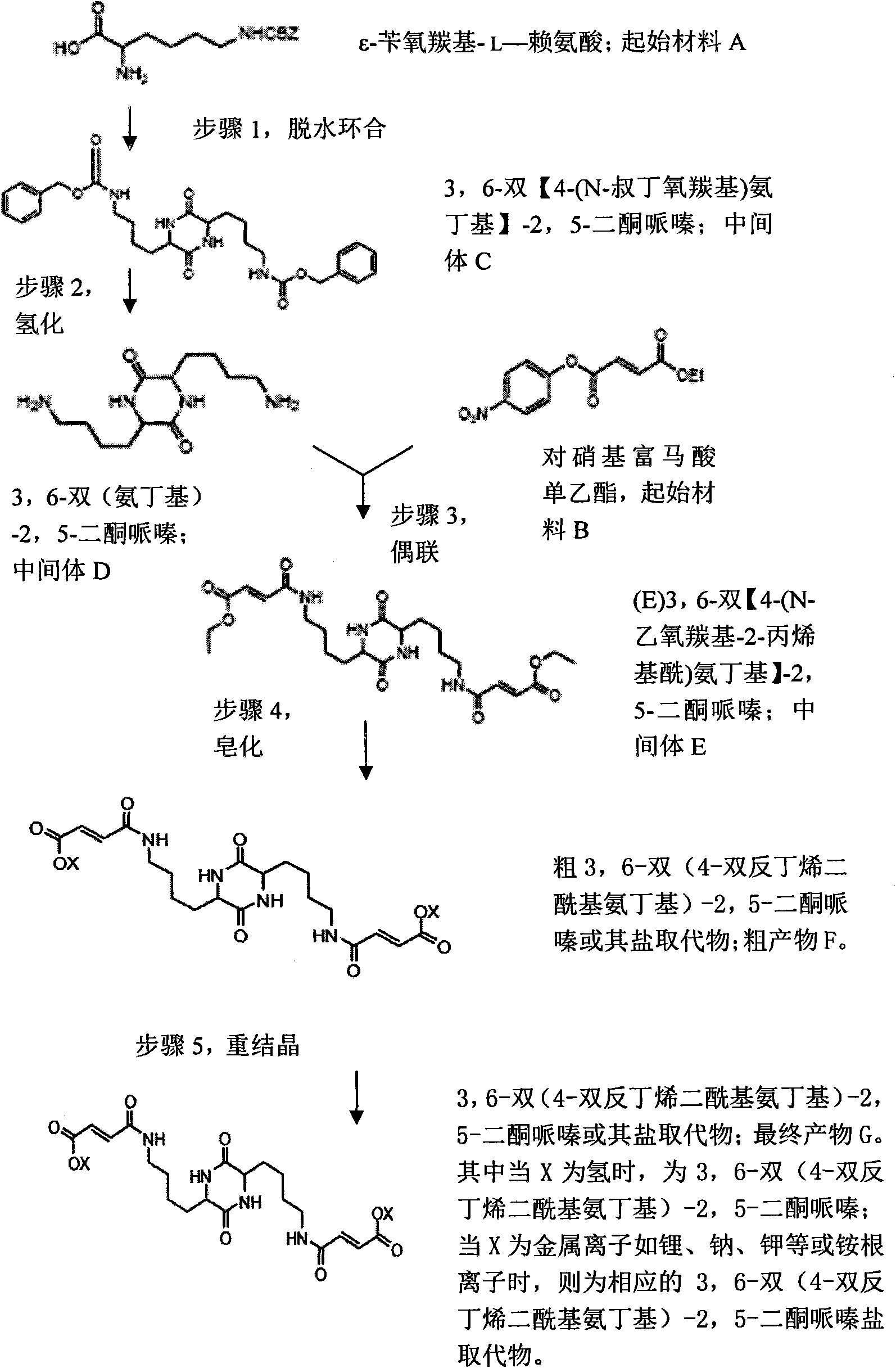
[0020] Embodiment 1: an instance of method 1;
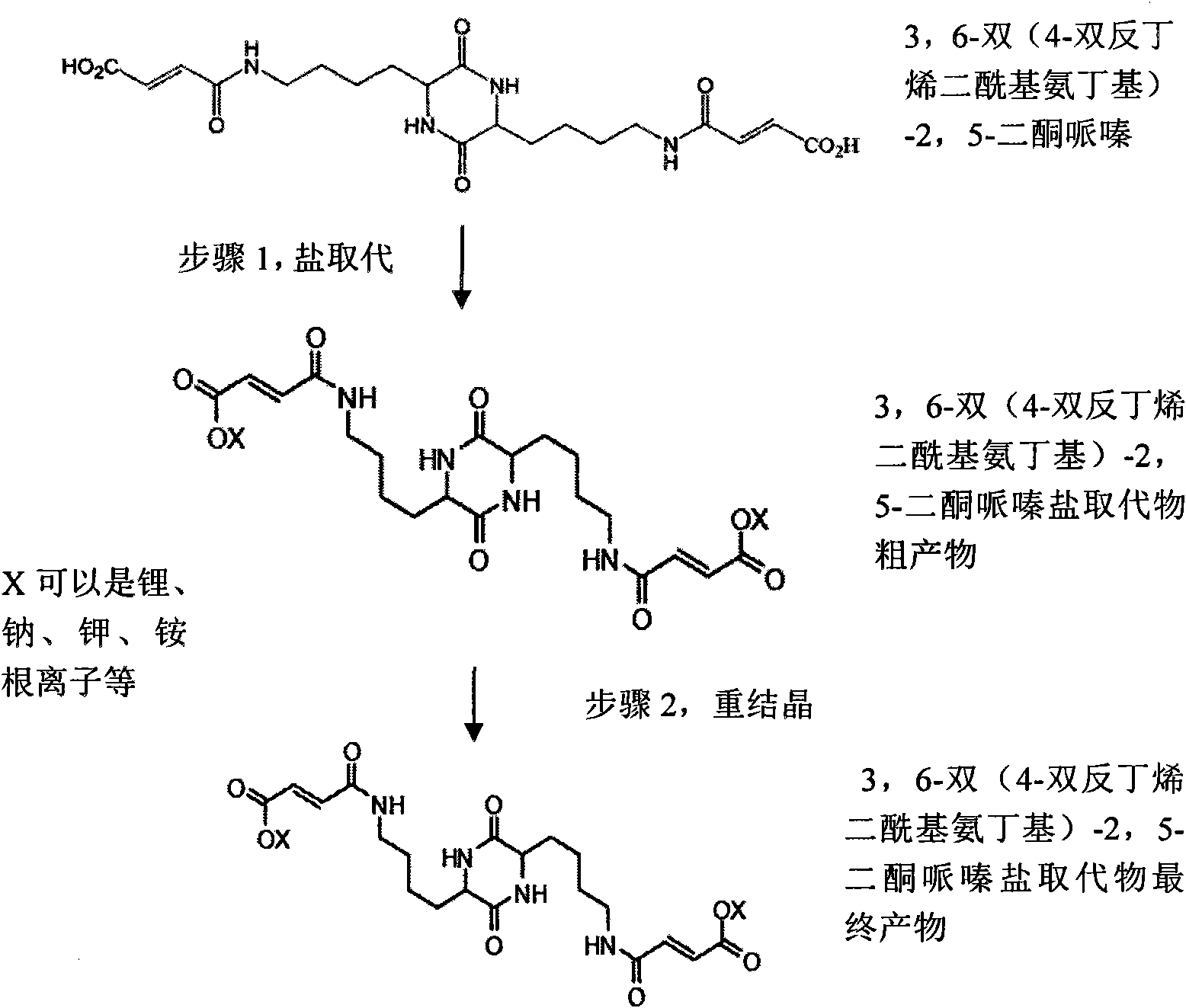
[0021] Step 1, dehydration ring closure reaction: add 30 grams of ε-benzyloxycarbonyl-L-lysine and 50 grams of m-cresol, and 5 grams of phosphorus pentoxide in a conical flask, heat to 200 ° C, during the reaction The water was distilled off, and then the reactant was cooled in a mixed solution of water and sodium hydroxide (10:1) to form a precipitate. After the precipitate was separated and washed with 30 ml of ethanol, the crude product of the intermediate 3,6-bis[4-(N-tert-butoxycarbonyl)aminobutyl]-2,5-diketopiperazine was obtained after filtration 20.6 grams. The obtained crude product was heated (100° C.) in 100 ml of glacial acetic acid solution, then added with 30 ml of pure water to cool, and then washed with 50 ml of glacial acetic acid solution to obtain the intermediate 3,6-bis[ 11.2 g of the refined product of 4-(N-tert-butoxycarbonyl)aminobutyl]-2,5-diketopiperazine. Step 2, hydrogenation reaction: 11.2 g of the...
Embodiment 2
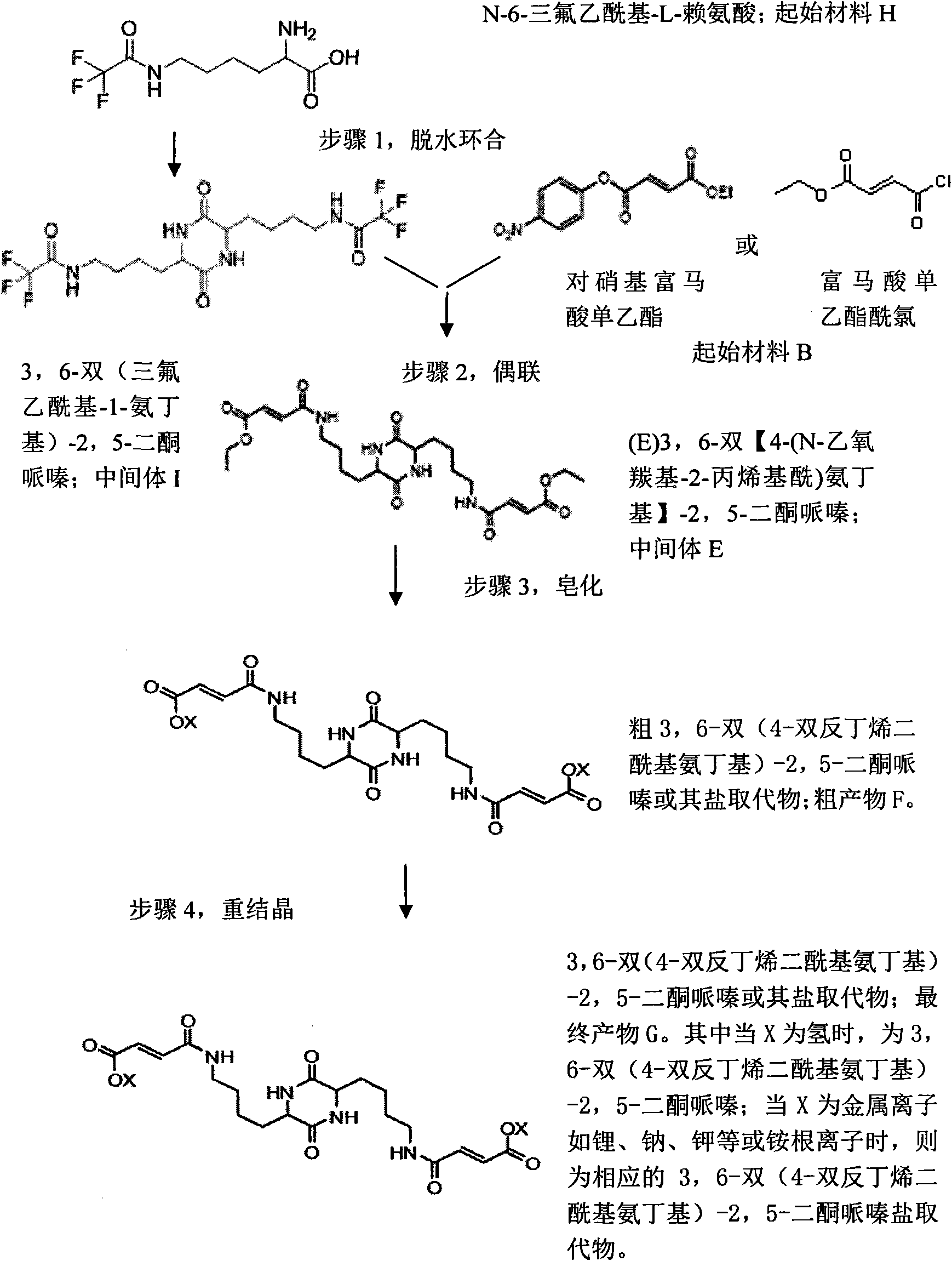
[0025] Embodiment 2: an example of method 2;
[0026]Step 1, dehydration ring closure reaction: add 30 grams of N-6-trifluoroacetyl-L-lysine, 30 grams of bis(2-methoxyethyl ether) and 5 grams of dioxypentoxide in an Erlenmeyer flask Phosphorus, mixed and stirred and then heated to 180°C. During the reaction, the water was removed by means of distillation, and then the reactant was cooled to room temperature in a mixed solution of water and sodium hydroxide (10:1) to form a precipitate. After the precipitate was separated and washed with 30 ml of ethanol, 18.5 g of crude intermediate 3,6-bis(trifluoroacetyl-1-aminobutyl)-2,5-diketopiperazine was obtained. Heat the obtained crude product to 130°C in 100 ml of glacial acetic acid solution, then add 20 ml of pure water to cool, then wash the crystals with 60 ml of glacial acetic acid solution, and then rinse with 60 ml of pure water to obtain the intermediate 12.8 g of the refined product of 3,6-bis(trifluoroacetyl-1-aminobutyl)-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com