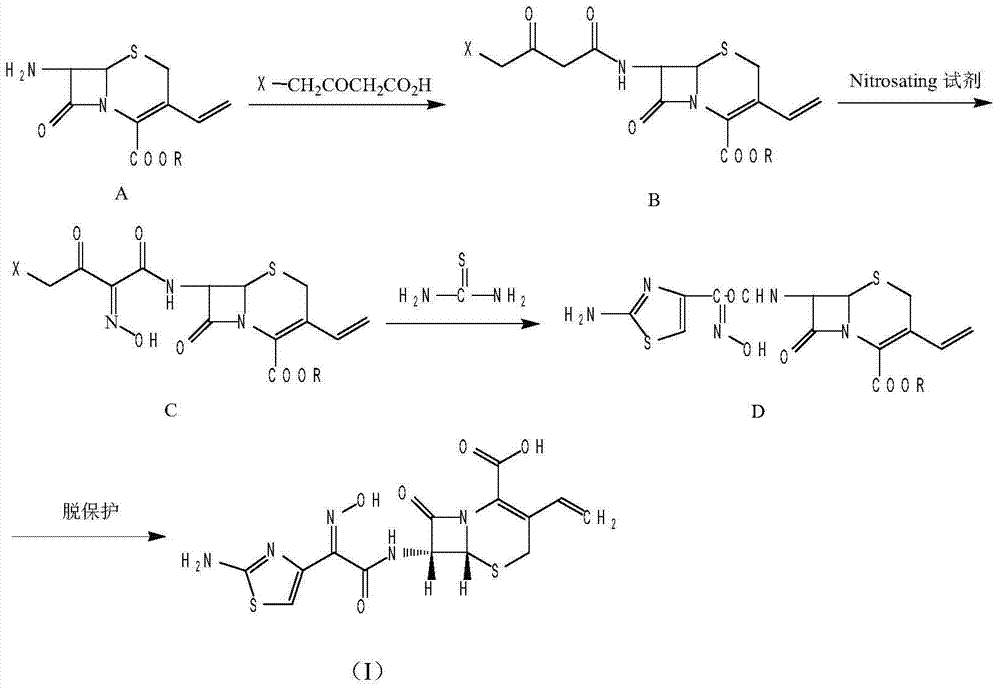
Method for preparing antibacterial cefdinir
A technology of cefdinir and acid anhydride, which is applied in the field of preparation of cefdinir to achieve the effects of simple reaction process, high yield and improved reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Preparation of mixed anhydride
[0032] Take (Z)-2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetic acid (demethylaminothioxamic acid) 100g (0.53mol), suspend and stir in 200ml dichloromethane, cool to -10℃ , dropwise added 81ml (0.56mol) of triethylamine, the temperature was controlled to be less than -5°C during the dropping process, and after the dropping was completed, a clear solution was obtained. Cool down to -15°C, add 128 g (1.12 mol) of methanesulfonyl chloride dropwise, after the drop is complete, stir and react at -10°C for 1 hour, set aside.
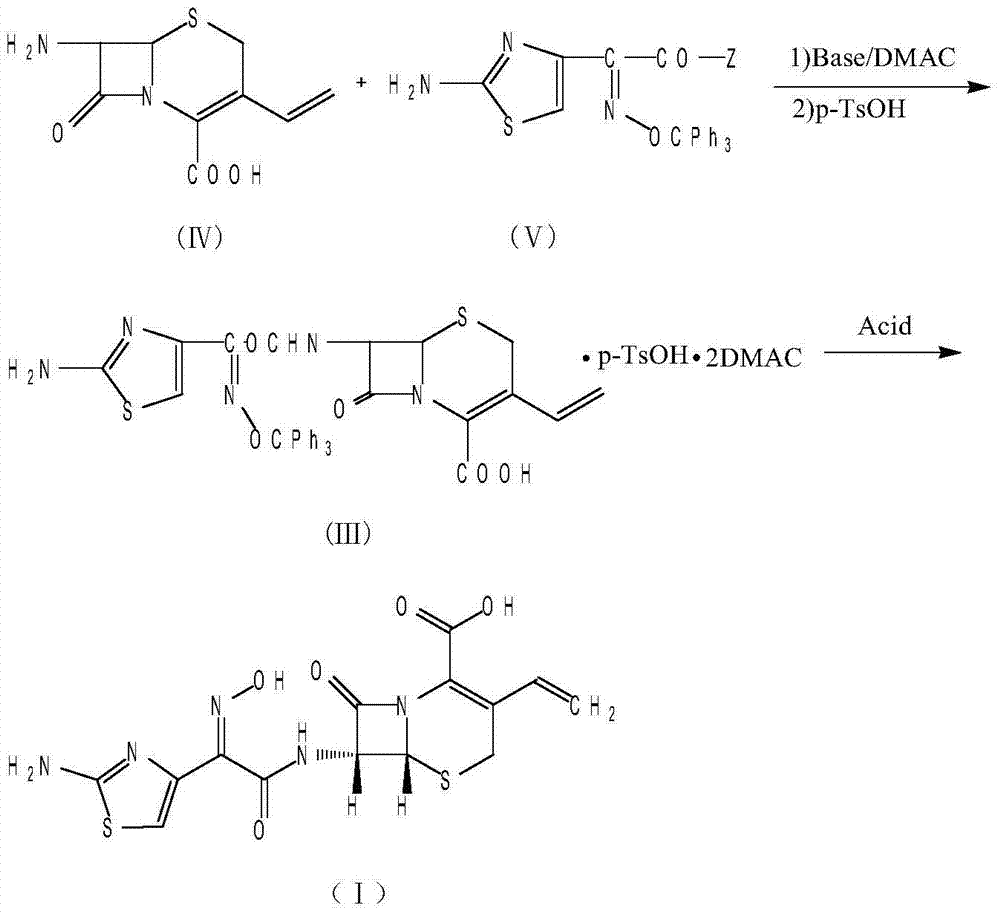
[0033] (2) (6R,7R)-7-[[(2-amino-4-thiazolyl)-(oximino)acetyl]amino]-3-vinyl-8-oxo-5-thia-1 - Preparation of azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (cefdinir)
[0034] Take 120g (0.53mol) of 7-amino-3-vinyl-3-cephem-4-carboxylic acid, suspend and stir in 2000ml of dichloromethane, cool down to -10°C, add dropwise 81ml of triethylamine (0.56mol ), after dropping, a clear solution was obtained. Add the mixed acid...
Embodiment 2
[0040] 1) Preparation of mixed anhydrides
[0041] Take (Z)-2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetic acid (demethylaminothioxamic acid) 100g (0.53mol), suspend and stir in 200ml dichloromethane, cool to -10℃ 134ml (0.56mol) of tri-n-butylamine was added dropwise, the temperature was controlled to be less than -5°C during the dropwise addition process, and a clear solution was obtained after the dropwise addition. Cool down to -15°C, add 128 g (1.12 mol) of methanesulfonyl chloride dropwise, after the drop is complete, stir and react at -10°C for 1 hour, set aside.
[0042] 2) (6R,7R)-7-[[(2-amino-4-thiazolyl)-(oximino)acetyl]amino]-3-vinyl-8-oxo-5-thia-1- Preparation of azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (cefdinir)
[0043]Take 120g (0.53mol) of 7-amino-3-vinyl-3-cephem-4-carboxylic acid, suspend and stir in 2000ml of dichloromethane, cool down to -10°C, add dropwise 134ml of tri-n-butylamine (0.56 mol), after dropping, a clear solution was obtained. Add the ...
Embodiment 3
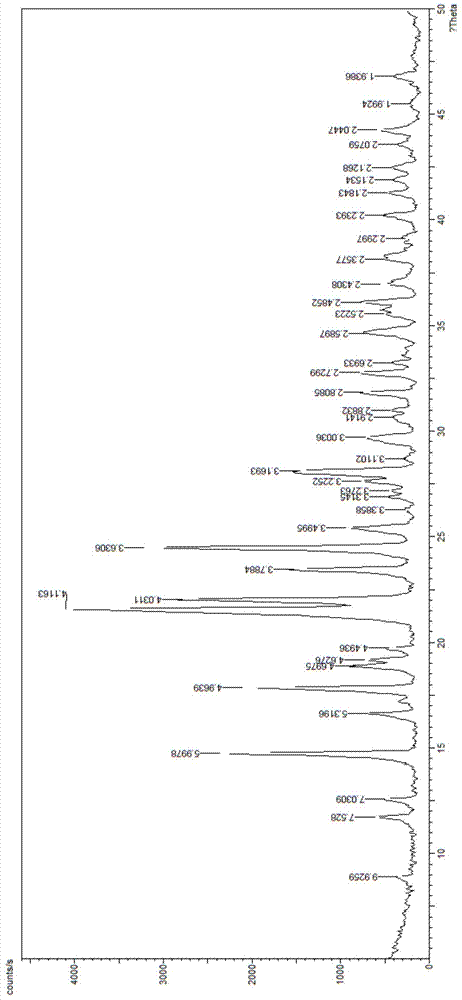
[0045] According to Example 1, dichloromethane was replaced with ethyl acetate to obtain 152.6 g (yield 72.8%) of cefdinir slightly yellow crystalline powder, with an HPLC purity of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com