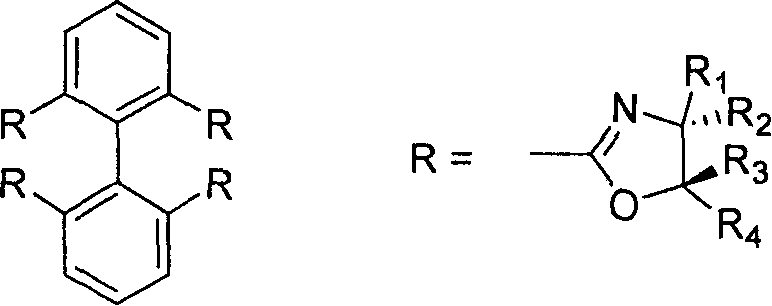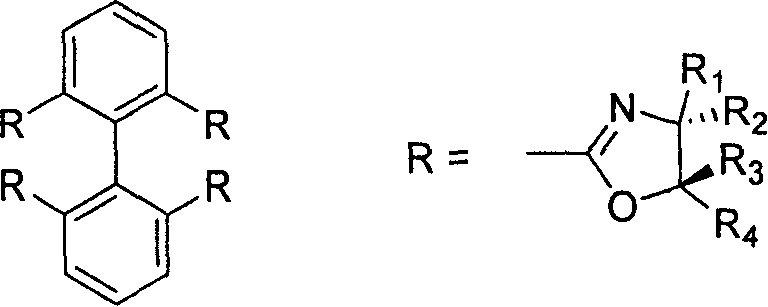2,2', 6,6'-tetraoxazoline diphenyl ligancy and preparation process thereof
A technology of tetraoxazoline biphenyl and ligand, applied in 2 fields, can solve problems such as waste of resources, and achieve the effects of simple synthesis method, good application prospect, high reactivity and stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Synthesis of compound I
[0022] Pyrene (3.00g, 14.90mmol) was dissolved in a solution of dichloromethane (60mL), acetonitrile (60mL) and water (100mL), and sodium periodate (29.94g, 140mmol) and trichloride were added to the above solution Ruthenium (120.31 g, 0.58 mmol). The reaction solution was heated to 40°C and stirred for 16h, a yellow precipitate formed. The solid obtained by filtration was dissolved in acetone (200 mL), and the insoluble matter was removed by filtration. The filtrate was concentrated by rotary evaporation to obtain white powder I (2.30 g, 76%).
[0023] 1 H NMR (400MHz, CD 3 COCD 3 ) 6.99 (d, J = 7.6 Hz, 4H, Ar-H), 6.77 (t, J = 7.6 Hz, 2H, Ar-H).
[0024] (2) Synthesis of compound II
[0025] Tetraacid I (1.00 g, 3.03 mmol) was dissolved in dichloromethane (20 mL), placed in an ice bath, and thionyl chloride (1.0 mL, 13.34 mmol) and 16 μLDMF were added to the solution. The temperature of the reaction solution was raised to room temp...
Embodiment 2
[0034] (1) Synthesis of compound I
[0035] The synthesis method refers to (1) in Example 1.
[0036] (2) Synthesis of compound II
[0037] The synthesis method refers to (2) in Example 1.
[0038] (3) Compound III (R 1 =i-Pr,R 2 = R 3 = R 4 =H) Synthesis
[0039] II (1.01 g, 2.49 mmol) was dissolved in dichloromethane (30 mL) and slowly added dropwise to a solution of L-valinol (1.97 g, 14.88 mmol) in dichloromethane (20 mL) under ice bath. After all was added dropwise, triethylamine (2.4 mL, 17.28 mmol) was added rapidly. After stirring at room temperature for 20 hours, wash with water and filter to obtain amido compound III (R 1 =i-Pr, R 2 = R 3 = R 4 =H) (1.44g, 2.14mmol), yield 86%.
[0040] (4) compound IV (R 1 =i-Pr, R 2 = R 3 = R 4 =H) Synthesis
[0041] Methanesulfonyl chloride (1.2 mL, 14.86 mmol) was added dropwise to amido compound III (R 1 =i-Pr,R 2 = R 3 = R 4 =H) (1.0 g, 1.49 mmol), triethylamine (2.5 mL, 17.67 mmol) and dichloromethane (10 mL...
Embodiment 3
[0043] (1) Synthesis of compound I
[0044] The synthesis method refers to (1) in Example 1.
[0045] (2) Synthesis of compound II
[0046] The synthesis method refers to (2) in Example 1.
[0047] (3) Compound III (R 1 =t-Bu, R 2 = R 3 = R 4 =H) Synthesis
[0048] II (1.00 g, 2.46 mmol) was dissolved in dichloromethane (40 mL) and slowly added dropwise to a solution of L-tert-butylleucinol (1.44 g, 12.30 mmol) in dichloromethane (20 mL) under ice bath. After all was added dropwise, triethylamine (1.8 mL, 12.96 mmol) was added rapidly. After stirring at room temperature for 20 hours, wash with water and filter to obtain amido compound III (R 1 =t-Bu, R 2 = R 3 = R 4 =H) (1.51 g, 2.08 mmol), yield 84%.
[0049] 1 H NMR (400MHz, CD 3 OD) 7.46-7.52 (m, 6H, Ar-H), 3.69-3.74 (m, 8H, NCH, OCH), 3.37 (dd, J=10.8, 12.8Hz, 4H, OCH), 0.734 (s, 36H, CH 3 ).
[0050] (4) compound IV (R 1 =t-Bu, R 2 = R 3 = R 4 =H) Synthesis
[0051] Methanesulfonyl chloride (0.5 mL, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com