Novel synthesizing method for preparing ursodesoxycholic acid (UDCA) from chenodeoxycholic acid
The technology of ursodeoxycholic acid and chenodeoxycholic acid is applied in the field of treating gallstones and preparing liver-protecting drugs, and can solve the problems of poor stereoselectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
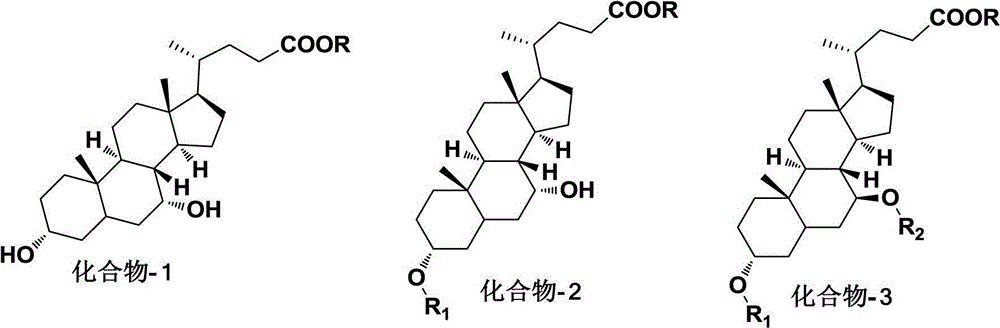
Examples
Embodiment 1
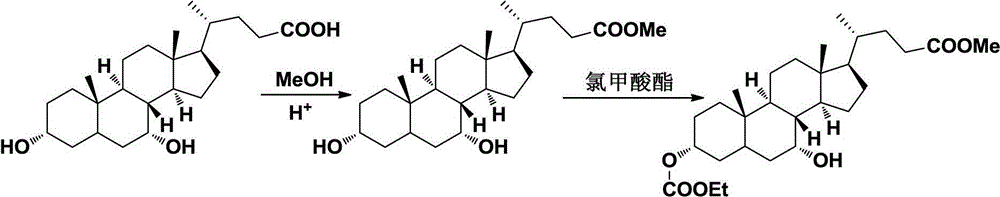
[0011] Synthesis of methyl chenodeoxycholic acid
[0012] Dissolve chenodeoxycholic acid (39.2g, 0.1mol) in 100ml of methanol, add 1ml of concentrated sulfuric acid, heat and reflux and stir for 10h, after the reaction is complete, after concentration, add 200ml of ethyl acetate and 100ml of water, adjust with saturated potassium carbonate solution pH to neutral, the organic layer was separated, washed with water, dried over anhydrous sodium sulfate, the solvent was recovered under reduced pressure, and recrystallized to obtain 37.2 g of a white solid, with a yield of 91.6%; mp: 156-157 °C; NMR (CDCl 3 300MHz) δppm 0.63(3H,s,C-18Me),0.94(3H,s,C-19Me),3.66(3H,s,COOMe),3.65(2H,brm,C-3 and C-7 C H OH) The rest are cholic acid mother nucleus peaks.
Embodiment 2
[0014] Synthesis of 3-ethylcarbonate-chenodeoxycholic acid methyl ester
[0015] Methyl chenodeoxycholic acid (20.3g, 0.05mol), 20ml of dichloromethane, 6ml of pyridine, 6g of ethyl chloroformate was added dropwise at 0-5°C, stirred at room temperature for 2h, diluted with water, extracted with dichloromethane, The organic layers were combined, washed successively with 10% hydrochloric acid and water, dried over anhydrous sodium sulfate, and the solvent was recovered under reduced pressure to obtain 17.6 g of an off-white solid with a yield of 73.6%; mp136-137°C; NMR (CDCl 3 300MHz) δppm: 0.66(3H,s,C-18Me),0.91(3H,s,C-19Me)1.28(3H,t,OCOCH 2 C H 3 )3.64(3H,s,COOMe)3.82(1H,m,C-7CHOH),4.14(2H,q,OCOC H 2 CH 3 ),4.44(1H,brm,C-3 CH OCOCH 2 CH 3 ) and the rest are cholic acid mother nucleus peaks.
Embodiment 3
[0017] Synthesis of 3-ethylcarbonate-7-methanesulfonate-chenodeoxycholic acid methyl ester
[0018] 3-Ethyl carbonate-chenodeoxycholic acid methyl ester (4.78g, 0.01mol) was dissolved in 5ml of pyridine, and methanesulfonyl chloride (8g, 0.011mol) was slowly added dropwise at room temperature, stirred overnight, and after the reaction was completed, pour In ice water, extract twice with 50ml dichloromethane, combine the organic layers, wash with 10% hydrochloric acid, 5% sodium bicarbonate solution, and water successively, dry over anhydrous sodium sulfate, recover the solvent under reduced pressure to dryness, and recrystallize to obtain prism 4.6g of needle-like crystals, yield 82.7%, mp107-108°C; NMR (CDCl 3 300MHz) δppm: 0.67 (3H, s, C-18Me), 0.94 (3H, s, C-19Me), 1.33 (3H, t, OCOCH2C H 3 ),3.04(3H,s,SO 2 Me), 3.61 (3H, s, COOMe), 4.17 (2H, q, OCOC H 2 CH 3 ),4.43(1H,brm,C-3 CH OCOEt), 4.89 (1H,brm,C-7 CH OSO 2 CH 3 ) and the rest are cholic acid mother nucleus ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com