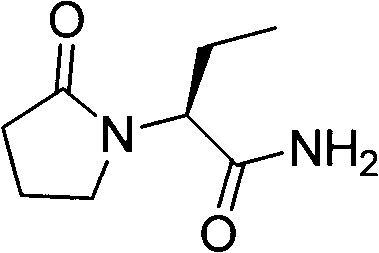
Novel synthetic method of antiepileptic drug levetiracetam
An antiepileptic drug, a new synthesis technology, applied in the field of chiral drug synthesis technology, can solve the problem of no reported analytical data on the chemical purity and optical purity of the final product, and achieve simple and easy reaction operations, high yields, and conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0032] Add LV40 (24 g, 0.14 mol) and dichloromethane (100 mL) into a 250 mL four-neck round bottom flask, stir and cool down to 0-5°C. Keep warm at -5~0°C, slowly add 26.7g (0.14mol) of p-toluenesulfonyl chloride dissolved in 50ml of dichloromethane dropwise, after 10±2min, stir the reaction for 25~30min, cool down to -5~0°C, Ammonia gas was started. Detect once with HPLC every 1 hour, after continuing to feed ammonia gas for 5 hours, HPLC shows that the content of raw material is less than 1.0%, stop feeding ammonia gas.
[0033] The reaction solution was filtered, the filter cake was washed with dichloromethane (15ml×3), and the filtrates were combined to obtain a light yellow clear solution. The solvent dichloromethane was evaporated under reduced pressure at 35-38°C to obtain crude levetiracetam.
[0034] Add 260ml of ethyl acetate to the crude product and heat to 60-65°C to dissolve. After complete dissolution and clarification, it was filtered while hot, and the filtr...
Embodiment example 2
[0036]Add LV40 (34.0 g, 0.2 mol) and dichloromethane (120 mL) into a 250 mL four-neck round bottom flask, stir and cool down to 0-5°C. Slowly add 24.0 g (0.21 mol) of methanesulfonyl chloride dropwise at 0-5°C under temperature control, after 10±2 minutes to complete the dripping, stir the reaction for about 30±5 minutes, cool down to 0-5°C, and start feeding ammonia gas. Detect once with HPLC every 1 hour, after continuing to flow ammonia gas for 6 hours, HPLC shows that the raw material content is less than 1.0%, stop passing ammonia gas.
[0037] The reaction solution was filtered, the filter cake was washed with dichloromethane (15ml×3), and the filtrate was combined. The filtrate was a slightly yellow clear liquid, and the solvent dichloromethane was rotary evaporated under reduced pressure at 35 to 38°C, and a white solid was gradually precipitated to obtain Etiracetam crude. Add 320ml of ethyl acetate to the crude product and heat to 60-65°C to dissolve. After complet...
Embodiment example 3
[0039] Add LV40 (34 g, 0.2 mol) and toluene (240 mL) into a 250 mL four-neck round bottom flask, stir and cool down to 0-5°C. Keep warm at -5~0°C, slowly add 24.0g (0.21mol) of methanesulfonyl chloride dropwise, 10±2min to complete the drop, stir for 25~30min, cool down to -5~0°C, and start to feed ammonia gas. Detect once with HPLC every 1 hour, after continuing to feed ammonia gas for 6 hours, HPLC shows that the content of raw material is less than 1.0%, stop feeding ammonia gas.
[0040] The reaction solution was filtered, the filter cake was washed with toluene (15ml×3), and the filtrates were combined to obtain a light yellow clear solution. The solvent toluene was rotary evaporated at 65-70°C under reduced pressure to obtain crude levetiracetam, which was added with 320ml of ethyl acetate and heated to 60-63°C to dissolve. After complete dissolution and clarification, it was filtered while hot, and the filtrate was cooled and crystallized slowly. Suction filtration af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com