Synthesis method of biochanin A
A technology of biochanin and a synthesis method, which is applied in the field of synthesis of biochanin A, can solve the problems of low yield and complex synthesis process, and achieve the effects of high yield, simple process and time saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
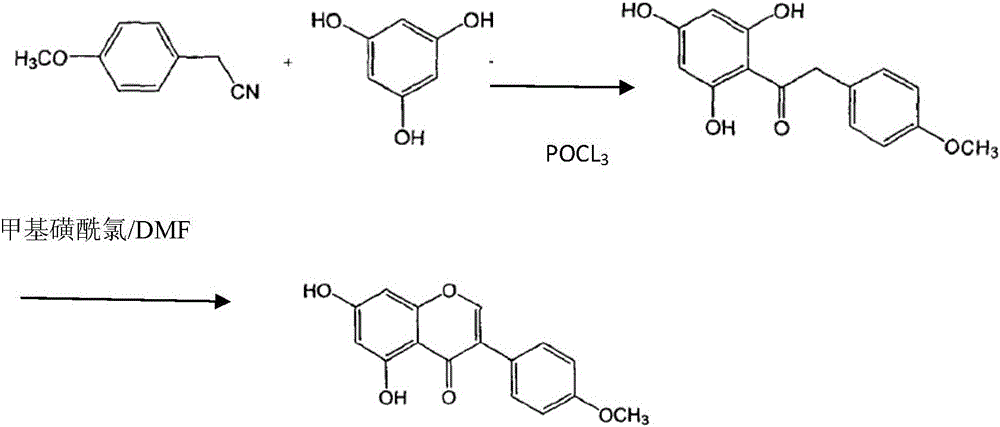
[0021] The first step: the synthesis of intermediates
[0022] In a 500 ml three-necked flask equipped with an electric stirrer, 37.8 g of anhydrous phloroglucinol, 51 g of p-methoxyphenylacetonitrile, and 100 ml of isopropyl ether were added. Start the electromagnetic stirrer at room temperature to fully stir for one hour, and then start to add 39.3 g of phosphorus oxychloride dropwise, and the dropwise addition is completed in 2 hours. After continuing to stir and react for 48 hours, slowly add 200 ml of water into the reaction liquid, reflux for 2 hours, add 2 g of activated carbon, continue to reflux for 30 minutes, and filter while hot. The filtrate was cooled and placed for 24h, and crystals were precipitated. Suction filtration, filter cake drying. 80 g of yellow needle-like product was obtained.
[0023] The second step: synthesis of biochanin A
[0024] Add 80 grams of the above-mentioned intermediate and 250 grams of DMF into a 500 ml three-necked reaction bottle...
Embodiment 2
[0028] The first step: the synthesis of intermediates
[0029] In a 250 ml three-necked flask equipped with an electric stirrer, 18.9 g of anhydrous phloroglucinol, 25.5 g of p-methoxyphenylacetonitrile, and 50 ml of isopropyl ether were added. Start the electromagnetic stirrer at room temperature and stir thoroughly for one hour, then start to drop 19.5 g of phosphorus oxychloride, and finish the dropwise addition in 2 hours. After continuing to stir and react for 48 hours, slowly add 100 ml of water into the reaction liquid, reflux for 2 hours, add 2 g of activated carbon, continue to reflux for 30 minutes, and filter while hot. The filtrate was cooled and placed for 24h, and crystals were precipitated. Suction filtration, filter cake drying. 38 g of yellow needle-like product was obtained.
[0030] The second step: synthesis of biochanin A
[0031] Add 38 grams of the above-mentioned intermediate and 125 grams of DMF into a 250 ml three-necked reaction bottle, add 52 gr...
Embodiment 3
[0035] The first step: the synthesis of intermediates
[0036] In a 1000 ml three-necked flask equipped with an electric stirrer, 76 g of anhydrous phloroglucinol, 103 g of p-methoxyphenylacetonitrile, and 200 ml of isopropyl ether were added. Start the electromagnetic stirrer at normal temperature to fully stir for one hour, then start to add 80 g of phosphorus oxychloride dropwise, and the dropwise addition is completed in 2 hours. After continuing to stir and react for 48 hours, slowly add 400 ml of water into the reaction solution, reflux for 2 hours, add 4 g of activated carbon and continue to reflux for 30 minutes, and filter while it is hot. The filtrate was cooled and placed for 24h, and crystals were precipitated. Suction filtration, filter cake drying. 156 g of yellow needle-shaped product was obtained.
[0037] The second step: synthesis of biochanin A
[0038] Add 156 grams of the above-mentioned intermediate and 500 grams of DMF into a 1000 ml three-necked rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com