Preparation process of insulin sensitizer
An insulin sensitizer and a technology for the preparation process are applied in the field of preparation technology of the insulin sensitizer, and can solve the problems that the preparation process lacks practical application value in industrialized production and the yield is relatively low.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
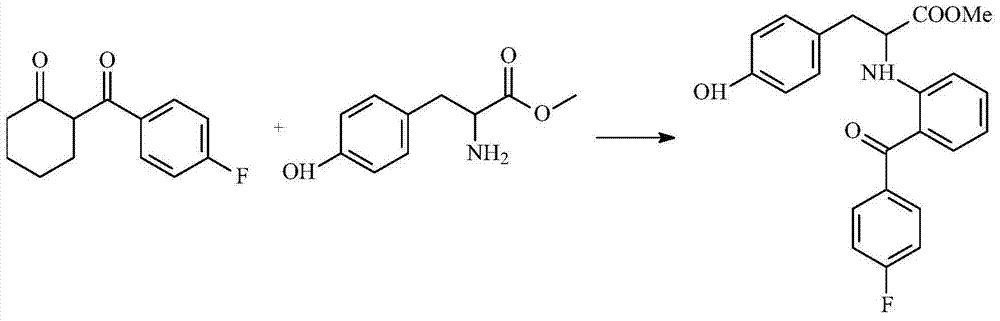
[0013] Embodiment 1: preparation 2-[2-(4-fluorobenzoyl) aniline]-3-(4-hydroxyphenyl)-propionic acid methyl ester
[0014]
[0015] Add 2.2 g of 2-(4-fluorobenzoyl) cyclohexanone, 1.96 g of L-tyrosine methyl ester, 30 ml of dioxane and 30 ml of toluene into a 100 ml reaction flask, and mix with an inert gas, such as Nitrogen was replaced three times, and the reflux reaction was performed for at least 5 hours to obtain the first reaction intermediate. After the reaction is complete, the first reaction intermediate is subjected to vacuum distillation, the purpose of which is to completely distill the solvent of the first reaction intermediate. Then, 50 ml of anisole and 1.5 g of palladium / carbon (Pd / C) with a concentration of 10% were added to the first reaction intermediate, and the reflux reaction was carried out for at least 3 hours. After the reaction was complete, the solvent of the first reaction intermediate was removed by distillation again, and the obtained crude pro...
Embodiment 2
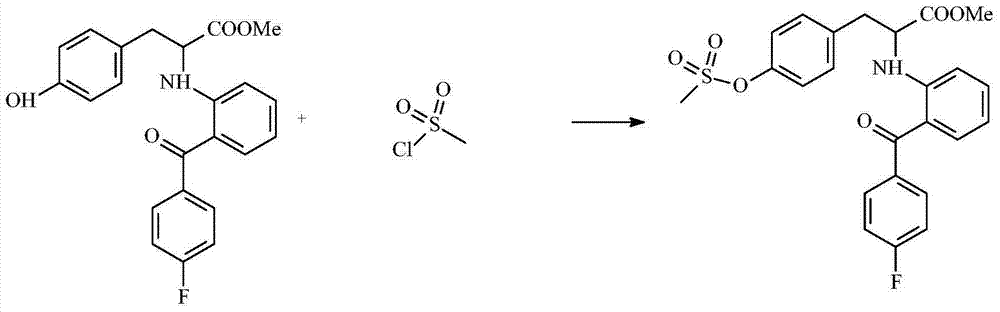
[0016] Embodiment 2: Preparation 2-[2-(4-fluorobenzoyl) aniline]-3-(4-methylsulfonyl phenyl)-propionic acid methyl ester
[0017]
[0018] Add the first product prepared in Example 1, 2-[2-(4-fluorobenzoyl)aniline]-3-(4-hydroxyphenyl)-propionic acid methyl ester, 3.93 g and 30ml of dichloromethane are mixed, and the mixture is cooled to 3 to 8 degrees, and the optimum cooling temperature is 5 degrees. Then, slowly drop 3.5 g of methanesulfonyl chloride and a small amount of pyridine into the mixed solution, keep the reaction temperature of the mixed solution at a cooling temperature, and carry out the synthesis reaction for at least 2 hours. It should be noted here that the "trace" in the dropwise addition of a trace amount of pyridine here refers to the amount of dropping one drop of pyridine after the dropper absorbs pyridine, which is called a trace amount. After the reaction, a second reaction intermediate is obtained, which is a liquid, and then the second reaction in...
Embodiment 3
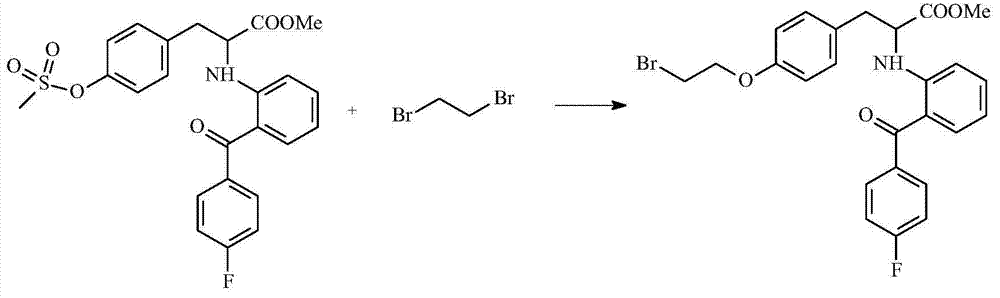
[0019] Embodiment 3: Preparation 2-[2-(4-fluorobenzoyl)aniline]-3-[4-(2-bromoethoxy)phenyl]-propionic acid methyl ester
[0020]
[0021]In a 100ml reaction flask, add the second product prepared in Example 2, 2-[2-(4-fluorobenzoyl)aniline]-3-(4-methylsulfonylphenyl)-propionic acid methyl Esters, 4.46g and solvent tetrahydrofuran 20ml, after stirring and dissolving, add 1.88g of 1,2-dibromoethane dropwise, and raise the reaction temperature to 45°C-55°C, the optimum reaction temperature is 50°C, and react here Synthesis is carried out at temperature for at least 2.5 hours to 3 hours. Obtain the 3rd reaction intermediate after reaction is complete, and this 3rd reaction intermediate is liquid, and it is diluted with the water of 100ml, makes it have a large amount of yellow solids to separate out in the process of diluting, then these separated yellow solids are separated out. The solid is dried to obtain the third product, 2-[2-(4-fluorobenzoyl)aniline]-3-[4-(2-bromoethoxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com